��Ŀ����
����Ŀ�����ȶ�ϵ������������Ư����ҵ���������ָ�ꡣ
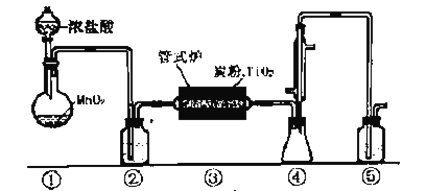
��.������ͼװ�ã�ʡ�Լ���װ�ã���̽��Ư�۵��ȷֽ���������ʾ�������ڳ�ʪ�����е�Ư���������ɵ����������O2������Cl2��
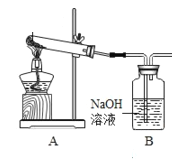
��1�����ȸ���Ư����Ʒ���۲쵽B���д�����ɫ���ݲ������������Ʒֽ�Ĺ��������һ����________��
��2�����Ⱦ����ڳ�ʪ�����е�Ư����Ʒ���۲쵽B��Ҳ�����ݲ�����
��B�з�����Ӧ�����ӷ���ʽΪ_______��
�ڴ���ַ�Ӧ�Ͽ�����A��B����Ƥ�ܣ�ֹͣ���ȣ���ȴ������B�в��ͨ��ʵ��֤������ʱ�������������ɡ������ʵ�鷽����_________��
��. �ⶨƯ�����ȵİٷֺ���������Ʒ����Ԫ������������Ʒ�������ı�ֵ����ʵ�鲽�����£�
��ȷ��ȡ5.000 gƯ����Ʒ����ϸ��������ˮ�ܽⲢ��ȴ��ϡ����500 mL��
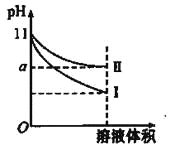
����ȡ25.00 mL��������Һ����ƿ�У�����pH��������������3% H2O2ˮ��Һ�����������ٲ������ݡ���������K2CrO4��Һ��Ϊָʾ������0.1000mol/L AgNO3����Һ�ζ����յ㡣���ʵ�飬�������AgNO3����Һƽ�����Ϊ25.00 mL������֪��Ksp(AgCl����ɫ)��1.56��10��10��Ksp(Ag2Cr2O4��ש��ɫ)��9.0��10��12��
��3��������У��ܽ⡢ϡ�͵Ĺ������õIJ����������ձ�����������____�� ____��
��4������H2O2ˮ��Һ�����������ٲ������ݣ�Ŀ����______�������ӷ���ʽ��ʾ����
��5���ζ��յ��������_______��
��6����Ư�����ȵİٷֺ���Ϊ__________��
��7�����в����������Ȱٷֺ����ⶨ���ƫ�ߵ���______��
A��ָʾ��K2CrO4����������
B���ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ��
C���ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
���𰸡� CaCl2 2OH��+Cl2��Cl��+ ClO��+ H2O ȡ����B����Һ���ȼ��������ữ���ٵμ���������Һ�����ְ�ɫ������˵����Cl2���� 500mL����ƿ ��ͷ�ι� ClO��+ H2O2��Cl��+O2��+ H2O ���������һ�α�Һ����Һ�г���ש��ɫ�������Ұ�����ڲ���ʧ 35.5% C
��������I. (1). ���ȸ����Ư����Ʒ���۲쵽B���д�����ɫ���ݲ�����˵��Ư���������ɵIJ�������O2��OԪ�صĻ��ϼ۴ӣ�2�����ߵ�0��������������ԭ��Ӧ�Ĺ��ɿ�֪��ClԪ�صĻ��ϼ�Ӧ��+1�۽��͵���1�������Դ�����Ʒֽ�Ĺ��������һ����CaCl2 ���ʴ�Ϊ��CaCl2 ��
(2). ��. ������ڳ�ʪ�����е�Ư���������ɵ����������O2������Cl2������B��Cl2��NaOH������Ӧ����NaCl��NaClO��H2O�����ӷ���ʽΪ��2OH��+Cl2��Cl��+ ClO��+ H2O���ʴ�Ϊ��2OH��+Cl2��Cl��+ ClO��+ H2O��
��. ��Ư�ۼ���ʱ�������������ɣ���������NaOH��Һ��Ӧ�����õ���Һ�к���Cl��������ֻҪ����B�к���Cl������֤����ʵ�鷽��Ϊ��ȡ����B����Һ���ȼ��������ữ���ٵμ���������Һ�����ְ�ɫ������˵����Cl2���ɣ��ʴ�Ϊ��ȡ����B����Һ���ȼ��������ữ���ٵμ���������Һ�����ְ�ɫ������˵����Cl2���ɣ�
II. (3). �����������������Ư����Ʒ��ϸ��������ˮ�ܽⲢ��ȴ��ϡ����500 mL��������IJ����������ձ����������⣬����Ҫ500mL����ƿ�ͽ�ͷ�ιܣ��ʴ�Ϊ��500mL����ƿ����ͷ�ιܣ�
(4). ����H2O2ˮ��Һ�����������ٲ������ݣ�Ŀ����ʹƯ����H2O2��ȫ��Ӧ���÷�Ӧ�����ӷ���ʽΪ��ClO��+ H2O2��Cl��+O2��+ H2O���ʴ�Ϊ��ClO��+ H2O2��Cl��+O2��+ H2O��
(5). ��AgNO3����Һ�ζ�������Һ�е�Cl����ȫ��Ӧ������ʼ����ש��ɫ��Ag2Cr2O4���������Եζ��յ������Ϊ�����������һ�α�Һ����Һ�г���ש��ɫ�������Ұ�����ڲ���ʧ����Ϊ�ζ��յ㣬�ʴ�Ϊ�����������һ�α�Һ����Һ�г���ש��ɫ�������Ұ�����ڲ���ʧ��
(6). �ɷ�Ӧ����ʽClO��+ H2O2��Cl��+O2��+ H2O��Ag����Cl��=AgCl����֪��ClO����Cl����Ag����������Ʒ����Ԫ�ص�������Ϊ��m(Cl)=0.025L��0.1mol/L��![]() ��35.5g/mol=1.775g�����Ư�����ȵİٷֺ���Ϊ��
��35.5g/mol=1.775g�����Ư�����ȵİٷֺ���Ϊ��![]() ��100%=35.5%���ʴ�Ϊ��35.5%��
��100%=35.5%���ʴ�Ϊ��35.5%��
(7). A����ָʾ��K2CrO4���������࣬��Һ��CrO42����Ũ�Ƚϴ����Cl����δ��ȫ����ʱ����ש��ɫ�������ɣ����ı���Һ�����ƫС���ⶨ���ƫ�ͣ���A����B���ڵζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ������ʹ��ȡ�ı���Һ���ƫС���ⶨ���ƫ�ͣ���B����C���ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ�����ʧ����ʹ����Һ���ƫ�ⶨ���ƫ������C��ȷ����ѡC��