��Ŀ����
����Ŀ��ij�¶�ʱ��Ag2SO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ�����¶��£�����˵����ȷ����
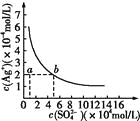
A. ���д���SO42-����Һ�п϶�������Ag+
B. 0.02 mol��L-1��AgNO3��Һ��0.02 mol��L-1��Na2SO4��Һ�������ϲ������ɳ���
C. Ag2SO4���ܶȻ�����(Ksp)Ϊ1��10-3
D. a���ʾAg2SO4�IJ�������Һ,��������ʹ��Һ��a��䵽b��
���𰸡�B
�����������������A��Ag2SO4��ˮ�г����ܽ�ƽ��Ϊ��Ag2SO4��s��2Ag+��aq��+SO42-��aq�����ܽ�Ϊ������̣���Һ��һ������Ag+��A����B��0.02mol/L��AgNO3��Һ��0.2mol/L��Na2SO4��Һ�������ϣ�c��SO42-��=0.1mol/Lʱ��c��Ag+��=0.01mol/L��c2��Ag+����c��SO42-��=1��10-5��ksp���������ɳ�����B��ȷ��C����ͼ���֪����c��SO42-��=5��10-2mol/Lʱ��c��Ag+��=2��10-2mol/L����ksp=[c��Ag+��] 2��[c��SO42-��]=2��10-5��C����D����������ʱ����Һ��Ag+��SO42-Ũ�ȶ�����D����ѡB��

��ϰ��ϵ�д�
�����Ŀ