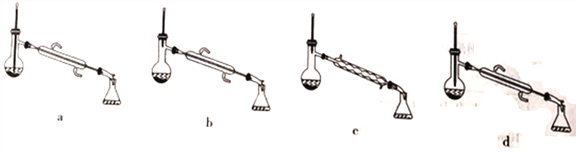
��Ŀ����
����Ŀ�����Ȼ��ѣ�TiCl4������ˮ�⣬�������е�ˮ���������������̡�����������Ļ�������۵㣭25�棬�е� 136.4�档ijʵ��С���������װ�ã����ּ��Ⱥͼг�װ��ʡ�ԣ�����Cl2��̿�ۡ�TiO2�Ʊ�TiCl4������˵������ȷ����

A. �ڡ����зֱ�ʢװ����ʳ��ˮ��NaOH��Һ
B. �������������������͵���������
C. ��Ӧ����ʱ��Ӧ��ֹͣ�۴����ȣ���ֹͣ�ٴ�����
D. ����ƴ��ڲ��㣬��ܢ�֮��ȱ�ٷ�ˮ��������ܵ�װ��
���𰸡�A
��������A. �����Ȼ��ѣ�TiCl4������ˮ�⣬�������е�ˮ�����������������������Ƶõ������к���ˮ���������Ԣ���ӦʢװŨ���ᣬ��A����B. TiCl4���۷е�ϵ����Ƶõ�TiCl4Ӧ�����������������װ���е������ܾ��������������͵��������ã���B��ȷ��C. ��Ӧ����ʱ����ֹͣ�۴����ȣ���ֹͣ�ٴ����ȣ�����ʹ�Ƶõ�TiCl4��������Χ����ȴ����ֹ��������װ����ʹTiCl4���ʣ���C��ȷ��D. �����Ȼ��ѣ�TiCl4������ˮ�⣬����Ӧ��װ�����͢�֮���һ����ֹˮ��������ܵ�װ�ã���D��ȷ����ѡA��

 ������������ϵ�д�
������������ϵ�д�����Ŀ����ҵ���ù������������ɴ��Ʊ�������������ԭ��(��ͼ��ʾ) ���й���������:
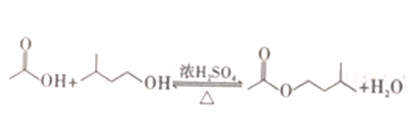
���� | ��Է������� | �ܶ�/(g��cm-3) | �۵�/�� | �е�/�� | ˮ���ܽ�� |
���촼 | 88 | 0.8123 | -117 | 131 | �� |
���� | 60 | 1.0492 | 17 | 118 | �� |
���������� | 130 | 0.8670 | -78 | 142 | ���� |
����˵����ȷ����
A. �����£����ù��˵ķ�������������������ˮ��Һ
B. ��Ӧ�У�����������������ҪĿ���Ǽӿ�÷�Ӧ�ķ�Ӧ����
C. Ϊ�˳�ȥ�����л��е����ᣬ�����������м�����������Na2CO3��Һ��������á���Һ
D. Ϊ�˳�ȥ�����л��е����촼��Ӧѡ����ͼ��ʾװ���е�cװ��