��Ŀ����
����Ŀ��ijУ��ѧ��ȤС��ͬѧ��������ˮ�п��ܺ��д���Ca2����Mg2����ijЩ�����ӣ��Ӷ�����������ʵ�飺
��ȡ��������ˮ���Թ��У��μ�������NaOH��Һ��������ɫ������
�ڹ��˺�ȡ��Һ���Թ��У��μ�������Na2CO3��Һ�����а�ɫ�������ɣ�
����ȡ��������ˮ���Թ��У��μ�����ϡ������ٵμ�AgNO3��Һ��Ҳ������ɫ������
��ش��������⣺
(1) ͨ��ʵ��ɳ���ȷ������ˮ��________(����������������������)����Ca2����Mg2����
(2) ����ˮ�����������ӿ���ȷ����___________����������AgNO3��Һ������Ӧ�����ӷ���ʽΪ________
���𰸡����� Cl-(��������) ![]()
��������
��1�����ݢ١��ڵ��������ɵİ�ɫ������̼��ƺ�������þ������ȷ������ˮ�к���Ca2+��Mg2+�����ӣ���Ӧ�����ӷ���ʽ�ǣ�CO32-+Ca2+=CaCO3����Mg2++2OH-=Mg(OH)2�����ʴ�Ϊ���У�
��2��Cl-+Ag+=AgCl������ɫ����������HNO3���μ�����ϡ������ٵμ�AgNO3��Һ��Ҳ������ɫ������˵������ˮ�к���Cl-���ʴ�ΪCl-(��������)��Cl-+Ag+=AgCl����

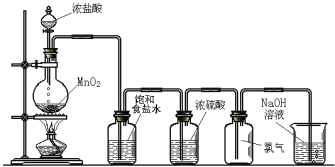
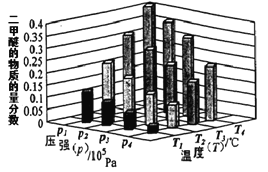
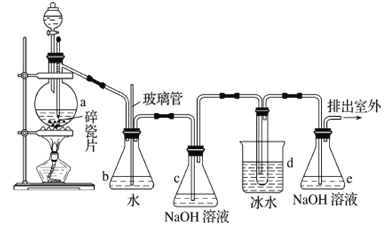
����Ŀ��1��2-����������������������Ӽ�����ͼΪʵ�����Ʊ�1��2-���������װ�Dͼ�� ͼ�з�Һ�ƶ�����ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ���dװ�D�Թ���װ��Һ�塣
��֪��CH3CH2OH![]() CH2=CH2��+H2O��2CH3CH2OH
CH2=CH2��+H2O��2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
��������б����£�
�Ҵ� | 1��2-�������� | ���� | �� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
�ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
�е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
�۵�/�� | -114.3 | 9.79 | - 116.2 | -7.2 |
ˮ���� | ���� | ���� | �� | ���� |
��1��ʵ����ӦѸ�ٽ��¶����l��170�����ҵ�ԭ����______________________________��
��2����ȫƿb��ʵ�����ж������á���һ���Լ��ʵ�������dװ�D�е����Ƿ���������
��д����������ʱƿb�е�����_______________________________�����ʵ��ʱdװ�D�е��ܶ���������Ϊ���ܵ�ԭ���Ǣ�_______________________________________________����ȫƿb�������������Ǣ�__________________��
��3������c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������_______________________________��
��4��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������ȷ����³������࣬���װ�D��������û�����⣬�Է������ܵ�ԭ��______________��______________��д���������ɣ���
��5����ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ___________��Ҫ��һ���ᴿ�����в����б������_____________ ������ĸ����
A���ؽᾧ B������ C����ȡ D������
��6��ʵ����Ҳ���Գ�ȥdװ�D��ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�D���Թ��У��� ��ʱ��ˮ����������ȴ1��2-��������������⣬��������������____________________________��