��Ŀ����
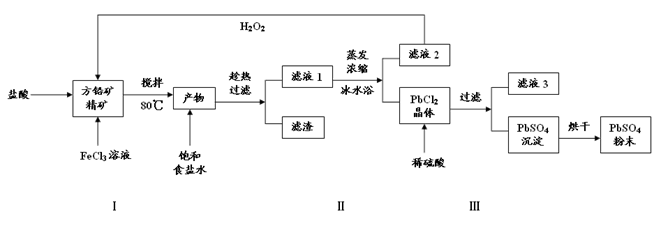
��16�֣�����Ǧ��PbSO4���㷺Ӧ��������Ǧ���ء���ɫ���ϵȡ����÷�Ǧ��PbS��ֱ���Ʊ�����Ǧ��ĩ���������£�
��֪��������PbCl2��s��+2Cl��(aq) PbCl4-(aq) ��H��0
PbCl4-(aq) ��H��0
������Ksp(PbSO4)=1.08��10-8, Ksp(PbCl2)=1.6��10-5
������Fe3����Pb2��������������ʽ��ȫ����ʱ����Һ��PHֵ�ֱ�Ϊ3.2��7.04
��1�������������PbCl2��S�����ӷ���ʽ �������������һ��Ŀ����Ϊ�˿���PHֵ��0.5��1.0��ԭ���� ��
��2���û�ѧƽ���ƶ���ԭ�����Ͳ������ʹ�ñ�ˮԡ��ԭ�� ��
��3��д��PbCl2����ת��ΪPbSO4���������ӷ���ʽ ��
��4���������ӷ���ʽ������Һ2����H2O2��ѭ�����õ�ԭ�� ����Һ3�� ��
��5��Ǧ���صĵ��Һ�����ᣬ���������缫�ϳ����� PbSO4�ֱ�ת��ΪPbO2��Pb�����ʱ�����ĵ缫��ӦʽΪ ��

��֪��������PbCl2��s��+2Cl��(aq)
 PbCl4-(aq) ��H��0
PbCl4-(aq) ��H��0������Ksp(PbSO4)=1.08��10-8, Ksp(PbCl2)=1.6��10-5
������Fe3����Pb2��������������ʽ��ȫ����ʱ����Һ��PHֵ�ֱ�Ϊ3.2��7.04
��1�������������PbCl2��S�����ӷ���ʽ �������������һ��Ŀ����Ϊ�˿���PHֵ��0.5��1.0��ԭ���� ��
��2���û�ѧƽ���ƶ���ԭ�����Ͳ������ʹ�ñ�ˮԡ��ԭ�� ��
��3��д��PbCl2����ת��ΪPbSO4���������ӷ���ʽ ��
��4���������ӷ���ʽ������Һ2����H2O2��ѭ�����õ�ԭ�� ����Һ3�� ��
��5��Ǧ���صĵ��Һ�����ᣬ���������缫�ϳ����� PbSO4�ֱ�ת��ΪPbO2��Pb�����ʱ�����ĵ缫��ӦʽΪ ��
��16�֣�
��1��PbS+2Fe3��+2Cl��= PbCl2+2Fe2��+S��3�֣�������Fe3����Pb2����ˮ�⣨2 �֣�
��2���ñ�ˮԡʹ��ӦPbCl2��s��+2Cl��(aq) PbCl4��(aq)�����ƶ���ʹPbCl4 ������ת��ΪPbCl2�������������3�֣�
PbCl4��(aq)�����ƶ���ʹPbCl4 ������ת��ΪPbCl2�������������3�֣�
��3��PbCl2��s��+SO42-(aq) PbSO4 (s) +2Cl��(aq) ��2�֣�
PbSO4 (s) +2Cl��(aq) ��2�֣�
��4��2Fe2��+ H2O2+2H��=2Fe3��+2H2O��2�֣������ᣨ2�֣�
��5��PbSO4+2e��="Pb+" SO42����2�֣�
��1��PbS+2Fe3��+2Cl��= PbCl2+2Fe2��+S��3�֣�������Fe3����Pb2����ˮ�⣨2 �֣�
��2���ñ�ˮԡʹ��ӦPbCl2��s��+2Cl��(aq)
 PbCl4��(aq)�����ƶ���ʹPbCl4 ������ת��ΪPbCl2�������������3�֣�
PbCl4��(aq)�����ƶ���ʹPbCl4 ������ת��ΪPbCl2�������������3�֣���3��PbCl2��s��+SO42-(aq)
 PbSO4 (s) +2Cl��(aq) ��2�֣�
PbSO4 (s) +2Cl��(aq) ��2�֣���4��2Fe2��+ H2O2+2H��=2Fe3��+2H2O��2�֣������ᣨ2�֣�
��5��PbSO4+2e��="Pb+" SO42����2�֣�
�����������1�������������PbCl2��S��˵����������ᡢ�Ȼ�����PbS����������ԭ��Ӧ��SԪ�ػ��ϼ����ߣ�����Ԫ�صĻ��ϼ۽��ͣ����ӷ���ʽΪPbS+2Fe3��+2Cl��= PbCl2+2Fe2��+S��Fe3����Pb2��������������ʽ��ȫ����ʱ����Һ��PHֵ�ֱ�Ϊ3.2��7.04�����Լ����������һ��Ŀ����Ϊ�˿���PHֵ��0.5��1.0��ԭ��������Fe3����Pb2����ˮ�⣻
��2�����뱥��ʳ��ˮ�����PbCl2��s��+2Cl��(aq)
 PbCl4-(aq) ��H��0���ڱ�ˮԡ�У��¶Ƚϵͣ�ʹƽ�������ƶ�������PbCl2�����������
PbCl4-(aq) ��H��0���ڱ�ˮԡ�У��¶Ƚϵͣ�ʹƽ�������ƶ�������PbCl2�������������3��Ksp(PbSO4)=1.08��10-8< Ksp(PbCl2)=1.6��10-5�����ݳ�����ת����PbCl2����ת��ΪPbSO4��������ΪPbCl2�м���ϡ�����ƻ����Ȼ�Ǧ���ܽ�ƽ�⣬ʹ�ܽ�ƽ�������ƶ���������Ǧ�����ӷ���ʽΪPbCl2��s��+SO42-(aq)
 PbSO4 (s) +2Cl��(aq)��
PbSO4 (s) +2Cl��(aq)����4����Һ2����Ҫ�ɷ����Ȼ���������������ⷴӦ�������Ȼ�������������ʹ�ã����ӷ���ʽΪ2Fe2��+ H2O2+2H��=2Fe3��+2H2O���ɣ�3����֪�Ȼ�Ǧת��Ϊ����Ǧͬʱ�����Ȼ��⣬������Һ3��������Һ��
��5�����ʱ�ǰѵ���ת��Ϊ��ѧ�ܣ��൱�ڵ���װ�ã���������������ԭ��Ӧ��Pb�Ļ��ϼ۽��ͳ�Ϊ����Pb���缫��ӦʽΪPbSO4+2e��="Pb+" SO42��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ



 MnO2+Zn +2H2SO4��
MnO2+Zn +2H2SO4��