��Ŀ����
ij���������̿�MnO2Լ70%��Al2 O3������п��ZnSԼ80%��FeS����ͬ����MnO2����Zn���ɵ��ԭ�ϣ����������£�

��֪����A��MnSO4��ZnSO4��Fe2(SO4)3��Al2(SO4)3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O MnO2+Zn +2H2SO4��
MnO2+Zn +2H2SO4��
��1��A�����ڻ�ԭ������� ��
��2������MnCO3��Zn2(OH)2CO3�������� ��C�Ļ�ѧʽ�� ��
��3���������г��õ�Na2SO4��S�ȸ���Ʒ�⣬���ɵõ��ĸ���Ʒ�� ��
��4������ƷS�����������ᣬת�������ǣ�S��SO2��SO3��H2SO4��д���ڶ���ת���Ļ�ѧ����ʽ ��
��5��Ҫ��Na2SO4��Һ�еõ�â���� Na2SO4��10H2O��������еIJ���������Ũ���� ��
���ˡ�ϴ�ӡ�����ȡ�
��6��������MnO2��Zn�ĽǶȼ��㣬���̿����п��Ͷ�ϵ������ȴ�Լ�� ��

��֪����A��MnSO4��ZnSO4��Fe2(SO4)3��Al2(SO4)3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O
 MnO2+Zn +2H2SO4��
MnO2+Zn +2H2SO4����1��A�����ڻ�ԭ������� ��
��2������MnCO3��Zn2(OH)2CO3�������� ��C�Ļ�ѧʽ�� ��
��3���������г��õ�Na2SO4��S�ȸ���Ʒ�⣬���ɵõ��ĸ���Ʒ�� ��
��4������ƷS�����������ᣬת�������ǣ�S��SO2��SO3��H2SO4��д���ڶ���ת���Ļ�ѧ����ʽ ��
��5��Ҫ��Na2SO4��Һ�еõ�â���� Na2SO4��10H2O��������еIJ���������Ũ���� ��
���ˡ�ϴ�ӡ�����ȡ�
��6��������MnO2��Zn�ĽǶȼ��㣬���̿����п��Ͷ�ϵ������ȴ�Լ�� ��
��1��MnSO4
(2)������Һ��p H,ʹFe3+��Al3+���ɳ��� H2SO4
��3��Fe2O3��Al2O3
(4) 2SO2+O2 2SO3
2SO3
(5) ��ȴ�ᾧ
��6��1��1(��1.03:1)
(2)������Һ��p H,ʹFe3+��Al3+���ɳ��� H2SO4
��3��Fe2O3��Al2O3
(4) 2SO2+O2
 2SO3
2SO3(5) ��ȴ�ᾧ
��6��1��1(��1.03:1)
����������Ƚ���Ϣ1��֪Mn���ϼ۽��ͣ����ڻ�ԭ�����ΪMnSO4���ɹ������̷�������MnCO3��Zn2(OH)2CO3��������������Һ��p H,ʹFe3+��Al3+���ɳ�������C�Ļ�ѧʽΪH2SO4�����ѵó��õ��ĸ���Ʒ����Fe2O3��Al2O3���������̿����п��������ֱ�Ϊx ��y�����ݢڿ�֪MnO2��Zn�����ʵ���֮��Ϊ1:1����0.7x g/87g/mol:0.8y g/97g/mol=1:1,���x/y=1.03:1.

��ϰ��ϵ�д�
�����Ŀ
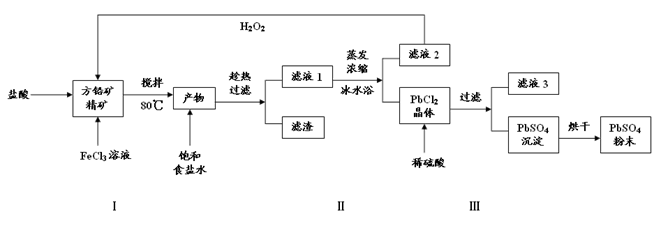
 PbCl4-(aq) ��H��0
PbCl4-(aq) ��H��0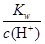 ��0.1 mol��L��1����Һ��Na����Cu2����HCO3����NO3��
��0.1 mol��L��1����Һ��Na����Cu2����HCO3����NO3��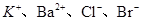
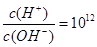 ����Һ�У�
����Һ�У� 
