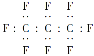ЬтФПФкШн
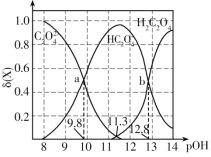
ЁОЬтФПЁПГЃЮТЯТЃЌПижЦЬѕМўИФБф0.1molЁЄL-1ЖўдЊШѕЫсH2C2O4ШмвКЕФpHЃЌШмвКжаЕФH2C2O4ЁЂHC2O4-ЁЂC2O42-ЕФЮяжЪЕФСПЗжЪ§ІФ(X)ЫцpOHЕФБфЛЏШчЭМЫљЪОЁЃвбжЊpOH=-lgc(OH-)ЃЌ![]() ЁЃЯТСаа№ЪіДэЮѓЕФЪЧЃЈ ЃЉ
ЁЃЯТСаа№ЪіДэЮѓЕФЪЧЃЈ ЃЉ
A.ЗДгІH2C2O4+C2O42-![]() 2HC2O4-ЕФЦНКтГЃЪ§ЕФжЕЮЊ103
2HC2O4-ЕФЦНКтГЃЪ§ЕФжЕЮЊ103
B.ШєЩ§ИпЮТЖШЃЌaЕувЦЖЏЧїЪЦЯђгв
C.pH=3ЪБЃЌ![]() =100.6ЃК1
=100.6ЃК1
D.ЮяжЪЕФСПХЈЖШОљЮЊ0.1molЁЄL-1ЕФNa2C2O4ЁЂNaHC2O4ЛьКЯШмвКжаЃКc(Na+)>c(C2O42-)>c(HC2O4-)>c(H+)>c(OH-)
ЁОД№АИЁПB
ЁОНтЮіЁП
AЃЎЗДгІH2C2O4+C2O42-![]() 2HC2O4-ЕФЦНКтГЃЪ§K=
2HC2O4-ЕФЦНКтГЃЪ§K=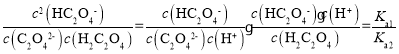 ЃЌaЕуc(C2O42-)= c(HC2O4-)ЃЌДЫЪБpOH=9.8ЃЌдђc(OH-)=10-9.8mol/LЃЌc(H+)=10-4.2mol/LЃЌдђKa2=
ЃЌaЕуc(C2O42-)= c(HC2O4-)ЃЌДЫЪБpOH=9.8ЃЌдђc(OH-)=10-9.8mol/LЃЌc(H+)=10-4.2mol/LЃЌдђKa2=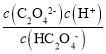 = 10-4.2ЃЛЭЌРэbЕуc(HC2O4-)=c(H2C2O4)ПЩЕУKa1=10-1.2ЃЌЫљвдЗДгІЕФЦНКтГЃЪ§K=
= 10-4.2ЃЛЭЌРэbЕуc(HC2O4-)=c(H2C2O4)ПЩЕУKa1=10-1.2ЃЌЫљвдЗДгІЕФЦНКтГЃЪ§K=![]() ЃЌЙЪAе§ШЗЃЛ
ЃЌЙЪAе§ШЗЃЛ
BЃЎЮТЖШЩ§ИпЃЌЫЎЕФЕчРыГЬЖШдіДѓЃЌЫЎЕФРызгЛ§БфДѓЃЌpHгыpOHжЎКѓаЁгк14ЃЌдђЭМЯёећЬхНЋЯђзѓвЦЖЏЃЌЙЪBДэЮѓЃЛ
CЃЎ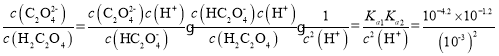 =100.6ЃК1ЃЌЙЪCе§ШЗЃЛ
=100.6ЃК1ЃЌЙЪCе§ШЗЃЛ
DЃЎH2C2O4ЕФKa1=10-1.2ЃЌKa2=10-4.2ЃЌдђHC2O4-ЕФЫЎНтЦНКтГЃЪ§Kh1=![]() ЃЌМДЦфЕчРыГЬЖШДѓгкЦфЫЎНтГЬЖШЃЌЫљвдДПNaHC2O4ШмвКжаc(C2O42-)>c(H2C2O4)ЃЌМгШыЕШЮяжЪЕФСПХЈЖШNa2C2O4ЁЂВнЫсФЦЛсЕчРыГіДѓСПЕФC2O42-ЃЌдђШмвКжаДцдкc(C2O42-)>c(HC2O4-)ЃЌОнЭМПЩжЊЕБc(C2O42-)>c(HC2O4-)ЪБШмвКГЪЫсадЃЌФЦРызгВЛЫЎНтЃЌЫљвдЛьКЯШмвКжаРызгХЈЖШДѓаЁЙиЯЕЮЊc(Na+)>c(C2O42-)>c(HC2O4-)>c(H+)>c(OH-)ЃЌЙЪDе§ШЗЃЛ
ЃЌМДЦфЕчРыГЬЖШДѓгкЦфЫЎНтГЬЖШЃЌЫљвдДПNaHC2O4ШмвКжаc(C2O42-)>c(H2C2O4)ЃЌМгШыЕШЮяжЪЕФСПХЈЖШNa2C2O4ЁЂВнЫсФЦЛсЕчРыГіДѓСПЕФC2O42-ЃЌдђШмвКжаДцдкc(C2O42-)>c(HC2O4-)ЃЌОнЭМПЩжЊЕБc(C2O42-)>c(HC2O4-)ЪБШмвКГЪЫсадЃЌФЦРызгВЛЫЎНтЃЌЫљвдЛьКЯШмвКжаРызгХЈЖШДѓаЁЙиЯЕЮЊc(Na+)>c(C2O42-)>c(HC2O4-)>c(H+)>c(OH-)ЃЌЙЪDе§ШЗЃЛ
ЙЪД№АИЮЊBЁЃ

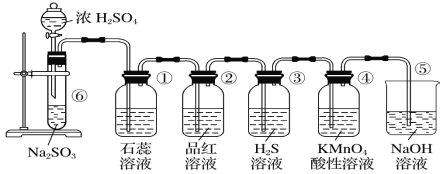
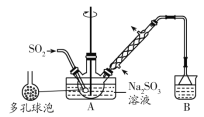
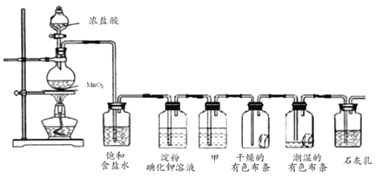
 ПЮЬУСЗМгВтЯЕСаД№АИ
ПЮЬУСЗМгВтЯЕСаД№АИ ЧсЫЩПЮЬУЕЅдЊВтЪдABОэЯЕСаД№АИ
ЧсЫЩПЮЬУЕЅдЊВтЪдABОэЯЕСаД№АИ