��Ŀ����
��ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ���ɫ�����������Ӱ�ˮ�������ܽ⣬�õ�����ɫ������Һ�������뼫�Խ�С���ܼ�(���Ҵ�)������������ɫ�ľ��塣
 ��һ���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�
��һ���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�
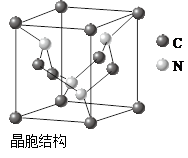
��1��д��H2O�Ŀռ乹��________��
��2��ˮ�������ض����������õ�һ��H+���γ�ˮ��������(H3O+)�����ж��������̵��������������� ��
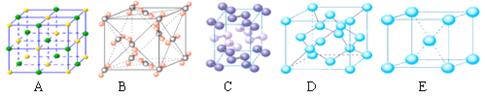
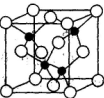
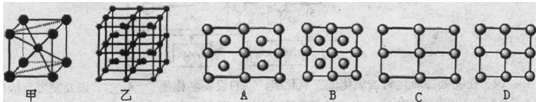
��3���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ʾ��ͼ(δ��˳������)������ľ���������ͬ���� (������Ӧ�ı����д)��
�������������������Ʋ�����Һ����Ҫԭ�ϣ�������Һ��һ�ֱ�����ɱ�������㷺Ӧ������ľ�������ͻ����ϡ�
��4��д��ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽ ����ͭͬһ���ڵĸ���Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ���� (��Ԫ�ط���)��
��5��ʵ��ʱ�γɵ�����ɫ��Һ�е��������ڴ��ڵ�ȫ����ѧ�������� ____ ��
��6��ʵ������м���C2H5OH��ɹ۲쵽��������ɫ[Cu(NH3)4]SO4��H2O���塣ʵ��������C2H5OH�������� ____________ ��
 ��һ���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�
��һ���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء���1��д��H2O�Ŀռ乹��________��
��2��ˮ�������ض����������õ�һ��H+���γ�ˮ��������(H3O+)�����ж��������̵��������������� ��
| A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
| C�����Ļ�ѧ���ʷ����˸ı� | D�����еļ��Ƿ����˸ı� |

�������������������Ʋ�����Һ����Ҫԭ�ϣ�������Һ��һ�ֱ�����ɱ�������㷺Ӧ������ľ�������ͻ����ϡ�
��4��д��ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽ ����ͭͬһ���ڵĸ���Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ���� (��Ԫ�ط���)��
��5��ʵ��ʱ�γɵ�����ɫ��Һ�е��������ڴ��ڵ�ȫ����ѧ�������� ____ ��
��6��ʵ������м���C2H5OH��ɹ۲쵽��������ɫ[Cu(NH3)4]SO4��H2O���塣ʵ��������C2H5OH�������� ____________ ��
��1�� V��(2��)�� ��2��A(2��)������3��BC(3��)
��4��3d104s1(2��)��;��Cr(2��)
��5�����ۼ�����λ��(2��) ��6������[Cu(NH3)4]SO4��H2O���ܽ��(2��)
��4��3d104s1(2��)��;��Cr(2��)
��5�����ۼ�����λ��(2��) ��6������[Cu(NH3)4]SO4��H2O���ܽ��(2��)
�����������һ����1��H2O�Ŀռ乹��ΪV�ͣ���2��A��ˮ�������ӻ�Ϊsp3��H3O+�������ӻ�Ϊsp3������ԭ�ӵ��ӻ�����û�иı䣬��������B��ˮ����ΪV�ͣ�H3O+Ϊ�����ͣ���������״�����˸ı䣬������C����ṹ��ͬ�������ʲ�ͬ�����Ļ�ѧ���ʷ����˸ı䣬������D��ˮ����ΪV�ͣ�H3O+Ϊ�����ͣ����еļ��Ƿ����˸ı䣬������ѡA����3�������ڷ��Ӿ��壬�ɾ���ʾ��ͼ��֪��AΪ�Ȼ��ƾ������������Ӿ��壬BΪ�ɱ��ľ��������ڷ��Ӿ��壬CΪ��ľ��������ڷ��Ӿ��壬DΪ���ʯ�ľ���������ԭ�Ӿ��壬CΪ�Ƶľ��������ڽ������壬������ľ���������ͬ����BC��
(��)��4��Cu��ԭ������Ϊ29��Ϊ�������ڵڢ�B��Ԫ�أ���۵����Ų�ʽΪ3d104s1����������Ϊ4s���ӣ�ͬһ���ڵĸ���Ԫ��Cr�ļ۵����Ų�Ϊ3d54s1����Ϊ��3d104s1��Cr����5��������Һ��������ˮ�γɵ�����ɫ��Һ�е�������Ϊ�İ���ͭ�����ӣ�N��Hԭ��֮���Թ��ۼ���ϣ�Cu2+�ṩ�չ����Nԭ���ṩ�¶Ե��ӣ�������λ����ϣ���Ϊ�����ۼ�����λ������6����5��������ɫ��Һ�м���C2H5OH��������Cu��NH3��4SO4?H2O���ܽ�ȣ���������ɫCu��NH3��4SO4?H2O���塣

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ����1����
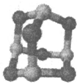
����1���� ����C6H6�ǷǼ��Է���
����C6H6�ǷǼ��Է���


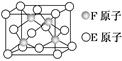
 ��2��CO��N2�Ľṹ�ɱ�ʾΪ��
��2��CO��N2�Ľṹ�ɱ�ʾΪ��