��Ŀ����
��ش��������⣺
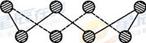
��1���������ڵ�ij����Ԫ�أ����һ�����������������ͼ1��ʾ�����Ԫ�ض�Ӧԭ�ӵ�M������Ų�ʽΪ ��
��2������ͼ2��ʾ��ÿ�����߱�ʾ���ڱ���A-��A�е�ijһ��Ԫ���⻯��ķе�仯��ÿ��С�ڵ����һ���⻯�����a��������� ����������ж����� ��
��3��CO2�ڸ��¸�ѹ�����γɵľ����侧������ͼ3��ʾ���þ������������ ��ѡ����ӡ���ԭ�ӡ������ӡ������������壬�þ�����̼ԭ�ӹ�����ӻ�����Ϊ ��

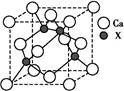
��4�������Ӿ��������������Ӽ������ܶ�ĽӴ����Խ�����ϵ��������ʹ�����ȶ����ڡ���֪Na+�뾶��Cl-��a����Cs+�뾶��Cl-��b������ع˿α���NaCl��CsCl�ľ������侧���߳���Ϊ ��
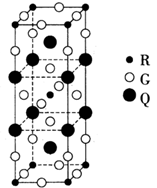
��5��Fe��һ�־�����ס�����ʾ�����������߷������ҵõ���A-Dͼ����ȷ���� ����ԭ�ӵ���λ���� ��������ԭ�ӵİ뾶��r cm���þ�����ܶ���p g��cm3�����������ԭ������Ϊ ���谢���ӵ�������ֵΪNA����

��1���������ڵ�ij����Ԫ�أ����һ�����������������ͼ1��ʾ�����Ԫ�ض�Ӧԭ�ӵ�M������Ų�ʽΪ ��
��2������ͼ2��ʾ��ÿ�����߱�ʾ���ڱ���A-��A�е�ijһ��Ԫ���⻯��ķе�仯��ÿ��С�ڵ����һ���⻯�����a��������� ����������ж����� ��
��3��CO2�ڸ��¸�ѹ�����γɵľ����侧������ͼ3��ʾ���þ������������ ��ѡ����ӡ���ԭ�ӡ������ӡ������������壬�þ�����̼ԭ�ӹ�����ӻ�����Ϊ ��

��4�������Ӿ��������������Ӽ������ܶ�ĽӴ����Խ�����ϵ��������ʹ�����ȶ����ڡ���֪Na+�뾶��Cl-��a����Cs+�뾶��Cl-��b������ع˿α���NaCl��CsCl�ľ������侧���߳���Ϊ ��
��5��Fe��һ�־�����ס�����ʾ�����������߷������ҵõ���A-Dͼ����ȷ���� ����ԭ�ӵ���λ���� ��������ԭ�ӵİ뾶��r cm���þ�����ܶ���p g��cm3�����������ԭ������Ϊ ���谢���ӵ�������ֵΪNA����

��1��3s23p6��2�֣�
��2��SiH4 ��1�֣����ڢ�A����A�е��⻯���NH3��H2O��HF����Ӽ����������ʷе����ͬ��������Ԫ���⻯��ķе㣬ֻ�Т�A��Ԫ���⻯�ﲻ���ڷ������������ṹ���ƣ���Է�����Խ���Ӽ�������Խ�е�Խ�ߣ�a���������߶�Ӧ������̬�⻯��SiH4��2�֣�
��3��ԭ�ӣ�1�֣� �� sp3�ӻ���2�֣�
��4�� (1+b)��
(1+b)��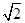 ��1+a�� ��2�֣�
��1+a�� ��2�֣�
��5��A ��2�֣��� 8 ��1�֣���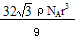 ��2�֣�
��2�֣�
��2��SiH4 ��1�֣����ڢ�A����A�е��⻯���NH3��H2O��HF����Ӽ����������ʷе����ͬ��������Ԫ���⻯��ķе㣬ֻ�Т�A��Ԫ���⻯�ﲻ���ڷ������������ṹ���ƣ���Է�����Խ���Ӽ�������Խ�е�Խ�ߣ�a���������߶�Ӧ������̬�⻯��SiH4��2�֣�
��3��ԭ�ӣ�1�֣� �� sp3�ӻ���2�֣�
��4��
 (1+b)��
(1+b)��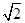 ��1+a�� ��2�֣�
��1+a�� ��2�֣���5��A ��2�֣��� 8 ��1�֣���
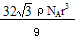 ��2�֣�
��2�֣������������1����Ԫ�ص���������ԶԶ���ڵڶ������ܣ�˵����Ԫ�ص�ԭ�Ӽ۵�����Ϊ2��ΪCa��M������Ų�ʽΪ��3s23p6
��2���ڢ�A����A�е��⻯���NH3��H2O��HF����Ӽ����������ʷе����ͬ��������Ԫ���⻯��ķе㣬ֻ�Т�A��Ԫ���⻯�ﲻ���ڷ������ڢ�A���γɵ��⻯���֮��Ϊ���»����������ṹ���ƣ���Է�����Խ���»���Խ�е�Խ�ߣ�����a���������߶�Ӧ������̬�⻯��SiH4��
��3����CO2�ڸ��¸�ѹ�����γɵľ���ͼ���Կ������侧��ṹΪ�ռ����ʽṹ��ÿ��Cԭ����Χͨ�����ۼ�����4��Oԭ�ӣ����Ըþ���Ϊԭ�Ӿ��壬̼ԭ�ӹ�����ӻ�����Ϊsp3�ӻ���
��4����Cl?�뾶Ϊr����Na+�뾶Ϊar��Cs+�뾶Ϊbr��NaCl�����߳�Ϊx����ΪNaCl����Ϊ���������ṹ������2x2=��2r+2ar��2����r=
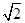 ��1+a��r��CsCl����Ϊ�����������ṹ������y2+2y2=��2r+2br��2���ɵ�y=2/
��1+a��r��CsCl����Ϊ�����������ṹ������y2+2y2=��2r+2br��2���ɵ�y=2/ (1+b)r��x��y=
(1+b)r��x��y= (1+b)��
(1+b)��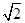 ��1+a��
��1+a����5������Feλ�ڶ�������ģ�����8������ɣ��������߷��������γɵ��ݽ���߳�����ȣ����ų�B��D������ÿ��С�����е����ĺ���1��Feԭ�ӣ���ӦΪA����ͼ���Կ�����λ�����ĵ���ԭ����Χ�����������ԭ����8����������ԭ�ӵ���λ����8����ͼ�����ı߳�Ϊacm����a2+2 a2=��4r��2����a=4
 /3r ��ͼ���������V=a3=64
/3r ��ͼ���������V=a3=64 /9r3�����ݾ�̯����֪���о�����Feԭ�ӣ�8��1/8+1=2,��Fe�����ԭ������ΪM����64
/9r3�����ݾ�̯����֪���о�����Feԭ�ӣ�8��1/8+1=2,��Fe�����ԭ������ΪM����64 /9r3?��="2M/" NA,M=
/9r3?��="2M/" NA,M=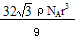

��ϰ��ϵ�д�
�����Ŀ
 Ϊ̼ԭ�ӣ�
Ϊ̼ԭ�ӣ�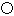 Ϊ��ԭ��)��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����________�����辧���߳�Ϊa cm���ܶ�Ϊb g��cm��3�����ӵ������ɱ�ʾΪ________(�ú�a��b��ʽ�ӱ�ʾ)��
Ϊ��ԭ��)��ÿ��̼ԭ����Χ�����������Ĺ�ԭ����________�����辧���߳�Ϊa cm���ܶ�Ϊb g��cm��3�����ӵ������ɱ�ʾΪ________(�ú�a��b��ʽ�ӱ�ʾ)��
 ��һ���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�
��һ���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮ��һ����õ��ܼ���ˮ������֮Դ���������ǵ�����������ء�


 2NH3ʵ�ִ�������⡣����˵����ȷ���� ������ѡ��
2NH3ʵ�ִ�������⡣����˵����ȷ���� ������ѡ��