��Ŀ����
����Ŀ�����ڱ�ǰ�����ڵ�Ԫ�� A��B��C��D��E��ԭ��������������A�ĺ����������������������ͬ��B��Dλ��ͬһ������δ�ɶԵ���������������������EΪ��������Ԫ�أ������ֻ��һ�����ӣ����������й�����������ӡ�
(1)B��C��D����Ԫ�ص�һ�������ɴ�С��˳��Ϊ___(��Ԫ�ط���)��E��̬ԭ�Ӽ۲�����Ų�ͼΪ_____��
(2)д��������Ԫ����ɵ�BD2�ĵȵ�����ķ��� _________��
(3)��֪D���γ�D3+���ӣ�����������ԭ���ӻ���ʽΪ___�����幹��Ϊ__��
(4)�¶Ƚӽ��е�ʱ��D�ļ��⻯���ʵ����������Ը�����ԭ�����ͻ�ѧʽ��������ķ�������ԭ���� _______��
(5)��ɫ��[E(CA3)2]+�ڿ����в��ȶ�������������������ɫ��[E (CA3)4]2+������������ʿɳ�ȥ�����е��������÷�Ӧ�����ӷ���Ϊ________��
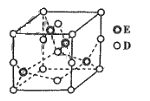
(6)��֪E��D�γɵ�һ�־�����ṹ��ͼ��ʾ����֪�����߳�Ϊanm�������ӵ�����ΪNA����þ�����ܶ�Ϊ_________ g/cm3(�г��������ʽ����)��
���𰸡�N��O��C ![]() N2O sp2 V�� ˮ�����дֵ�ˮ������Ϊ�������ϣ��γɵϷ��� 4[Cu(NH3)2]++O2+8NH3H2O=4[Cu(NH3)4]2++4OH-+6H2O
N2O sp2 V�� ˮ�����дֵ�ˮ������Ϊ�������ϣ��γɵϷ��� 4[Cu(NH3)2]++O2+8NH3H2O=4[Cu(NH3)4]2++4OH-+6H2O ![]()
��������
A�ĺ����������������������ͬ����A��HԪ�أ�B��Dλ��ͬһ������δ�ɶԵ��������������������������߶�Ϊ�������ڣ�δ�ɶԵ�����Ϊ3������������Ԫ�ص�������ֻ��һ�������������⣬��B��DΪ�ڶ����ڣ��������2��δ�ɶԵ��ӣ���2p2��2p4������BΪCԪ�أ�DΪOԪ�أ���CΪNԪ�أ�EΪ��������Ԫ�أ������ֻ��һ�����ӣ����������й�����������ӣ���E��CuԪ�ء�
(1)ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ�������Ԫ�أ���C��N��O�ĵ�һ�����ܴ�С��ϵΪ��N��O��C��CuΪ29��ԭ�ӣ���������Ų�ʽΪ[Ar]3d104s1����۵����Ų�ͼΪ![]() ��
��
(2)BD2ΪCO2������3��ԭ�ӣ��۵�����Ϊ16���ȵ�������ָԭ��������ȣ��۵���������ȵ���������������Ԫ����ɵ���CO2��Ϊ�ȵ�����ķ���ΪN2O��
(3)DΪOԪ�أ�����D3+����ΪO3+��������ԭ�ӵļ۲���Ӷ���Ϊ2+![]() =2.5������3���㣬����Ϊsp2�ӻ����µ��Ӷ���Ϊ1���������幹��ΪV�Σ�
=2.5������3���㣬����Ϊsp2�ӻ����µ��Ӷ���Ϊ1���������幹��ΪV�Σ�
(4)�¶Ƚӽ�ˮ�ķе��ˮ�����д��ڴֵ�ˮ������Ϊ�������ϣ��γɵϷ��ӣ�������ⶨֵƫ��
(5)��ɫ��[Cu(NH3)2]+�ڿ����в��ȶ�������������Ϊ����ɫ��[Cu(NH3)4]2+�����������������÷�ӦӦ�ڰ�ˮ�н��У����Ԫ���غ��֪�ù����л�����������ˮ���ɣ����ӷ���ʽΪ4[Cu(NH3)2]++O2+8NH3H2O=4[Cu(NH3)4]2++4OH-+6H2O��
(6))Cu��O�γ�һ�־��壬�þ�����Cuԭ�Ӹ���=4��Oԭ�Ӹ���=8��![]() +6��
+6��![]() =4�����Ծ���������Ϊ
=4�����Ծ���������Ϊ![]() g���þ������V=(a��10-7 cm)3����þ����ܶ�
g���þ������V=(a��10-7 cm)3����þ����ܶ� ��
��

����Ŀ���ڹ�ҵ����������β���к��ж��ֵ��������Ҫ��NO ��NO2������NOx����ʾ��NOx���ƻ������㣬�����⻯ѧ����������ɴ�����Ⱦ����Ҫ��Դ֮һ���ش��������⣺
(1)��֪ 1mol ���ӷֽ�Ϊ����ԭ������Ҫ������Ϊ�����ʡ� N2(g)��NO(g)��O2(g)�Ľ����ʷֱ�Ϊ941.7��631.8��493.7(��λkJ/mol)�����㷴Ӧ 2NO(g) = N2(g) + O2(g)����H=_______kJ/mol�����ж�NO(g)���¡���ѹ���ܷ��Է��ֽ� ________���ܻ���)��
(2)Ϊ��ֹ�⻯ѧ���������ӹ�������������ƽ��иĽ��⣬ҲҪ����ijЩ��ѧ�������ý�̿��ԭNOx �ķ�ӦΪ2NOx(g) + xC(s)N2(g) + xCO2(g)
��.�ں��������£�2 molNO2(g)������ C(s)��Ӧ�����ƽ��ʱ NO2(g)�� CO2(g)�����ʵ���Ũ����ƽ����ѹ�Ĺ�ϵ��ͼ��ʾ��
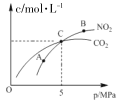
��A��B����NO2ƽ��ת���ʵĹ�ϵ��(A)____��(B)��ƽ�ⳣ����ϵK(A)_____K(B)(�������)��
�ڼ���C��ʱ�÷�Ӧ��ѹǿƽ�ⳣ��Kp=_____MPa(Kp����ƽ���ѹ����ƽ��Ũ��)���㣬��ѹ=��ѹ�����ʵ�������)��
��.�������ݻ���Ϊ 2L �������ܱ�����A��B�м���һ������ NO(g)��������C(s)����ͬ�¶��²����������n(NO)��ʱ��仯��������ʾ��
0 | 20 | 40 | 60 | 80 | |
n(NO)/mol(A) | 2 | 1.5 | 1.1 | 0.8 | 0.8 |
n(NO)/mol(B) | 1 | 0.8 | 0.65 | 0.53 | 0.45 |
B�����ڷ�Ӧ�� 100sʱ�ﵽƽ��״̬����0~100s���� NO ��ʾ��ƽ����Ӧ����Ϊv(NO)= ____________��
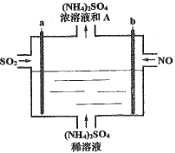
(3)�������绯ѧ�����ڴ����������﷽��Ҳ����һ�������ã���ͼ��һ�ְ���һ��������ȼ�ϵ�أ������¿ɽ���������ת��Ϊ������
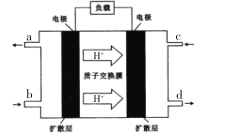
��c��ͨ�������Ϊ______ ��д��������Ӧ�ķ���ʽ ________��
����a��d�ڲ��������������Ϊ1.568L(�����)����·��ͨ���ĵ�����Ϊ____��
����Ŀ��������Ҫ��Ԫ�أ����γɶ��ֺ����ᡣ�ش���������:
(1)������(H3PO2)��һԪ�ᣬ����볣����ֵK=9��103����10mL0.1 molL-1H3PO2��Һ�м���30mL�����ʵ���Ũ�ȵ�NaOH��Һ��д����Ӧ�����ӷ���ʽ_________��c(Na+)+(H2PO2-)+c(H3PO2)=______(���Ի�Ϻ��ܲ�����ı仯)��
(2)������(H3PO3)�Ƕ�Ԫ���ᣬ 25��ʱ������ĵ��볣����ֵΪK1=1��10-2��k2=2.6��10��7,��NaH2PO3��Һ����_____(��ᡱ������С�)��ԭ����____(��ϻ�ѧ���P���ݼ�����н���)
(3)��֪HF�ĵ��볣����ֵΪK=3.6��10-4,������HF��Һ��Na2HPO3��Һ��Ӧ�������ӷ���ʽΪ______��
(4)���������ǿ��ԭ�ԡ���ѧʵ��С�����õζ����ⶨij��������Һ��Ũ�ȣ�ȡ25.00mL����������Һ������ƿ�У���0.10 molL-1�ĸ��������Һ���еζ�����Ӧ�����ӷ���ʽ��5H3PO3+ 2MnO4-+6H+ = 5H3PO4+ 2Mn2+ +3H2O��
���εζ�ʵ������ݷֱ�����:
ʵ���� | �ζ�ǰ���� | �ζ������ |
1 | 0.50 | 22.50 |
2 | 1.50 | 24.50 |
3 | 1.00 | 22.00 |
����������Һ�����ʵ���Ũ��Ϊ______��
�ڹ��ڸ�ʵ������˵����ȷ����______(��д���)��
a ȡ��������Һ�ĵζ��ܣ�ϴ�Ӻ�δ��ϴ�����½��ƫ��
b ʢ���������Һ�ĵζ��ܵζ�ǰ�����ݣ��ζ��������ݣ����½��ƫ��
c �ζ��������۾�ֻע�ӵζ�����Һ��仯�������ü�¼
d ��ƿδ����ײ���ˮ���ᵼ�½��ƫ��