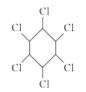
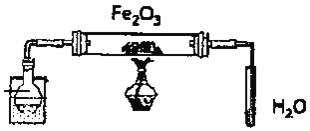��Ŀ����
����Ŀ���йش����Ĵ�����������ɴ����Ҵ�������ʵ�����õ�һЩ��ʶ����ͼ��ij��ѧ��ȤС����Ƶ��Ҵ���������ʵ��װ����(��ʾ��ͨ�������Ƶ�Cu(OH)2����Һ����ש��ɫ���������������к���ȩ��)��

���ͼ�ش��������⣺
��1���������Ӱ�װ��ϣ�����ʵ��ǰ��μ���װ�õ������ԣ���_____________��
��2����A�е��Ҵ�����ˮԡ���ȵ�Ŀ����____________��
��3��ʵ��ʱ����ȼB���ľƾ��ƺ���____________���ټ��л������ͭ˿�������װ���в��ϵػ��������������ʱB�й۲쵽��������____________����������Ҫ��Ӧ�Ļ�ѧ����ʽΪ____________������Ӧ����һ��ʱ�����ȥ�ƾ��ƣ��������ϻ����ع��������B�����ظ���������˵��B�������ķ�Ӧ��һ��____________��Ӧ(��������������������)��
��4��װ��C��������____________�����ڴ˴��۲쵽��������____________��
��5��װ��D�е�������____________��
���𰸡�
��1���رջ���K2����K1����ȼB���ƾ��ƣ�A��Һ��ѹ�볤���У�ֹͣ������ȴ�����£�A�лָ�ԭ״(��ر�K1����K2����ȼB���ƾ��ƣ�D�г��ܿ�ð���ݣ�ֹͣ������ȴ�����£�D��Һ�嵹���볤���γ�һ��ˮ��)��˵�����������ã�
��2��ΪʹA���Ҵ��ϳ�ʱ���ƽ�ȵ��������Ҵ�������
��3��Ԥ��ֱ������ ͭ˿�ɺ�ɫ��Ϊ��ɫ���ܿ��ֱ�Ϊ��ɫ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O ���ȣ�
2CH3CHO+2H2O ���ȣ�
��4�������Ҵ�����������H2O ��ɫ��ĩ��Ϊ��ɫ���壻
��5������ש��ɫ����
��������
�����������1��װ�������Լ����ԭ���ǣ�ͨ�����巢������Һ�幹�ɷ����ϵ�����ݸı���ϵ��ѹǿʱ����������(�����ݵ����ɡ�ˮ�����γɡ�Һ���������)���ж�װ�������Եĺû�������Ϊ���رջ���K2����K1����ȼB���ƾ��ƣ�A��Һ��ѹ�볤���У�ֹͣ������ȴ�����£�A�лָ�ԭ״(��ر�K1����K2����ȼB���ƾ��ƣ�D�г��ܿ�ð���ݣ�ֹͣ������ȴ�����£�D��Һ�嵹���볤���γ�һ��ˮ��)��˵�����������ã��ʴ�Ϊ���رջ���K2����K1����ȼB���ƾ��ƣ�A��Һ��ѹ�볤���У�ֹͣ������ȴ�����£�A�лָ�ԭ״(��ر�K1����K2����ȼB���ƾ��ƣ�D�г��ܿ�ð���ݣ�ֹͣ������ȴ�����£�D��Һ�嵹���볤���γ�һ��ˮ��)��˵�����������ã�
��2����A�е��Ҵ�����ˮԡ���ȵ�Ŀ����ΪʹA���Ҵ��ϳ�ʱ���ƽ�ȵ��������Ҵ��������ʴ�Ϊ��ΪʹA���Ҵ��ϳ�ʱ���ƽ�ȵ��������Ҵ�������
��3���Ҵ������������У�ͭ�����������ȷ�Ӧ��������ͭ������ͭ�����Ҵ���Ӧ������ȩ��ͭ��ˮ������ʵ��ʱ����ȼB���ľƾ��ƺ���Ԥ��ֱ�����ܣ��ټ��л������ͭ˿������������Ϊ��˿�ɺ�ɫ��Ϊ��ɫ���ܿ��ֱ�Ϊ��ɫ����Ӧ����ʽ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O����ȥ�ƾ��ƣ��������ϻ����ع��������B�����ظ���������˵���÷�ӦΪ���ȷ�Ӧ���ų��������ܹ�ά�ַ�Ӧ�Ľ��У��ʴ�Ϊ��Ԥ��ֱ������ ͭ˿�ɺ�ɫ��Ϊ��ɫ���ܿ��ֱ�Ϊ��ɫ��2CH3CH2OH+O2
2CH3CHO+2H2O����ȥ�ƾ��ƣ��������ϻ����ع��������B�����ظ���������˵���÷�ӦΪ���ȷ�Ӧ���ų��������ܹ�ά�ַ�Ӧ�Ľ��У��ʴ�Ϊ��Ԥ��ֱ������ ͭ˿�ɺ�ɫ��Ϊ��ɫ���ܿ��ֱ�Ϊ��ɫ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O ���ȣ�
2CH3CHO+2H2O ���ȣ�
��4������ͭΪ��ɫ���壬�����ˮ������ɫ����ˮ����ͭ��ͨ���ô˼���ˮ�Ĵ��ڣ��ʴ�Ϊ�������Ҵ�����������H2O ��ɫ��ĩ��Ϊ��ɫ���壻
��5����ȩ����ȩ���ܹ������Ƶ�������ͭ��Ӧ����ש��ɫ��������ͭ�������ˮ�����Կ���������Ϊ������ש��ɫ�������ʴ�Ϊ������ש��ɫ����

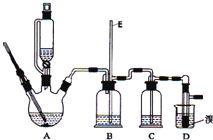
����Ŀ��ʵ����������������������Ҵ��Ʊ�1��![]() ���������װ����ͼ��ʾ��
���������װ����ͼ��ʾ��
��ʾ���Ҵ���Ũ������![]() ʱ��ˮ�������ѣ���
ʱ��ˮ�������ѣ���![]() ʱ��ˮ������ϩ���й������б����£�
ʱ��ˮ������ϩ���й������б����£�
�Ҵ� | 1�� | ���� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
�ܶ� | 0.79 | 2.2 | 0.71 |
�е� | 78.5 | 132 | 34.6 |
�۵� | -130 | 9 | -116 |
�ش��������⣺
![]() װ��D�з�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ ______
װ��D�з�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ ______
![]() װ��B������ ______ ����������E������ ______
װ��B������ ______ ����������E������ ______
![]() ��װ��C��Ӧ���� ______ ����Ŀ�������շ�Ӧ�п������ɵ���������
��װ��C��Ӧ���� ______ ����Ŀ�������շ�Ӧ�п������ɵ���������![]() ����ȷѡ��ǰ����ĸ
����ȷѡ��ǰ����ĸ![]()
![]() ˮ
ˮ![]() Ũ����
Ũ����![]() ����������Һ
����������Һ![]() ����̼��������Һ
����̼��������Һ
![]() ��Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ���� ______ �����ֲ��ܹ�����ȴ
��Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ���� ______ �����ֲ��ܹ�����ȴ![]() ���ñ�ˮ
���ñ�ˮ![]() ����ԭ���� ______ ����1��
����ԭ���� ______ ����1��![]() ��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ�� ______ ��
��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ�� ______ ��![]() ����������������
����������������![]()
![]() ��������������δ��Ӧ��
��������������δ��Ӧ��![]() ������� ______ ϴ�ӳ�ȥ
������� ______ ϴ�ӳ�ȥ![]() ����ȷѡ��ǰ����ĸ
����ȷѡ��ǰ����ĸ![]()
![]() ˮ
ˮ![]() ����������Һ
����������Һ![]() �⻯����Һ
�⻯����Һ![]() �Ҵ�
�Ҵ�