��Ŀ����
��11�֣�ijʵ��С���ͬѧΪ̽��һ�����������ʣ���ѡ������ʵ��װ������ȡһ��������
A B C D
��ش��������⣺
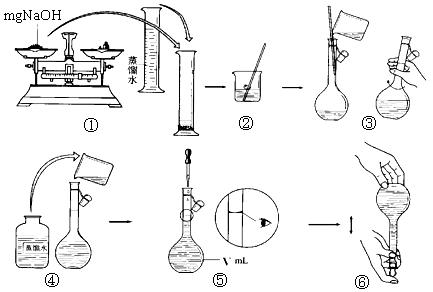
��1������ͼ����ʾ��װ����ȡһ��������ѡ���������ӵ�˳��Ӧ��A��_________��
��2��������15 mol/L HNO3��Һ����500 mL��1 mol/L HNO3��Һʱ��Ҫ�õ����������ձ�����ͷ�ιܡ�����������Ͳ�⣬������ʹ�õ�һ�ֲ��������� ��
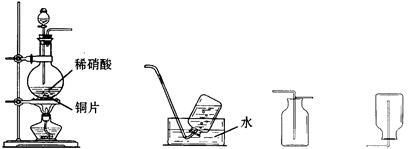
��3��ϡ�������ǿ�����ԣ��ڳ����¾Ϳ��Ժ�ͭ������Ӧ����һ���������壬д���÷�Ӧ�Ļ�ѧ����ʽ��___________________________________________________��
��4��ʵ�鿪ʼʱ����С��ͬѧ��������ƿ���к���ɫ��������������û�ѧ����ʽ����ʾ�������������ԭ��_________________________________________________��
��5��һ��������һ����̼������β���е���Ҫ��Ⱦ�Ŀǰ������β���ѳ�Ϊ�������п�������Ҫ��ȾԴ����������β���е�NO��CO��һ�ַ����ǣ�����������������װһ����ת��װ�ã�ʹNO��CO��Ӧ������CO2��N2����Ӧ�Ļ�ѧ����ʽ�� ��

A B C D
��ش��������⣺
��1������ͼ����ʾ��װ����ȡһ��������ѡ���������ӵ�˳��Ӧ��A��_________��
��2��������15 mol/L HNO3��Һ����500 mL��1 mol/L HNO3��Һʱ��Ҫ�õ����������ձ�����ͷ�ιܡ�����������Ͳ�⣬������ʹ�õ�һ�ֲ��������� ��
��3��ϡ�������ǿ�����ԣ��ڳ����¾Ϳ��Ժ�ͭ������Ӧ����һ���������壬д���÷�Ӧ�Ļ�ѧ����ʽ��___________________________________________________��
��4��ʵ�鿪ʼʱ����С��ͬѧ��������ƿ���к���ɫ��������������û�ѧ����ʽ����ʾ�������������ԭ��_________________________________________________��
��5��һ��������һ����̼������β���е���Ҫ��Ⱦ�Ŀǰ������β���ѳ�Ϊ�������п�������Ҫ��ȾԴ����������β���е�NO��CO��һ�ַ����ǣ�����������������װһ����ת��װ�ã�ʹNO��CO��Ӧ������CO2��N2����Ӧ�Ļ�ѧ����ʽ�� ��
��1��B (2)����ƿ��3��3Cu +8HNO3==3Cu(NO3)2 +2NO +4H2O
��4��2NO +O2==2NO2��5��2NO+2CO==2CO2+N2
��4��2NO +O2==2NO2��5��2NO+2CO==2CO2+N2
�����������1��NO������ˮ����������е�������Ӧ����ֻ������ˮ���ռ����������ӵ�˳��Ӧ��A��B��
��2���������ʵ���Ũ��һ������Һ���貣���������ձ�����ͷ�ιܡ�����������Ͳ������ƿ�ȡ��ʻ�����ʹ�õ�һ�ֲ�������������ƿ��
��3��ϡ�����ͭ��Ӧ����ʽ��3Cu +8HNO3==3Cu(NO3)2 +2NO +4H2O
��4����ƿ���к���ɫ�����������ΪNO��װ����ԭ�е�������Ӧ����NO2��Ӧ����Ӧ����ʽ2NO +O2==2NO2
��5���������ⷴӦ����ʽΪ��2NO+2CO==2CO2+N2
���������⿼����NO����ȡԭ�����ռ����������ʣ�������Һ�����ơ��ۺ��Խ�ǿ��

��ϰ��ϵ�д�
�����Ŀ