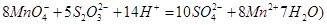��Ŀ����
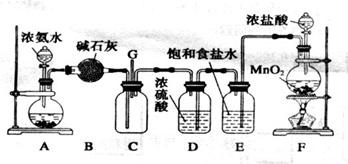
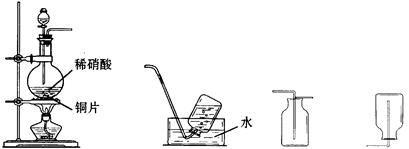
��11�֣�ij��ѧ��ȤС����������װ��̽�������백��֮��ķ�Ӧ������A��F�ֱ�Ϊ�����������ķ���װ�ã�CΪ��������������백��������Ӧ��װ�á�
��ش��������⣺
��1��װ��A�еĹ������ʲ���ѡ��_______________������ĸ��ţ���
a.��ˮ�Ȼ��� b.�������� c.������ d.��ʯ�� e.��ˮ����ͭ
��2��д��װ��F�з�����Ӧ�����ӷ���ʽ��
_______________________________________________________________________��
Eװ�õ�����Ϊ________________________________________________________��
��3��ͨ��Cװ�õ�����������߽ϳ����ұ߽϶̣�ԭ����________________________
___________________________________________________________________________��
��4��װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ������0.15molCl2���뷴Ӧʱ�������ɵ������ڱ�״���µ����Ϊ____________��
��5������װ�û�����һ�����Ե�ȱ�ݣ�����Ϊ�Ľ��Ĵ�ʩ��____________________
___________________________________________________________________________��

��ش��������⣺
��1��װ��A�еĹ������ʲ���ѡ��_______________������ĸ��ţ���
a.��ˮ�Ȼ��� b.�������� c.������ d.��ʯ�� e.��ˮ����ͭ
��2��д��װ��F�з�����Ӧ�����ӷ���ʽ��
_______________________________________________________________________��
Eװ�õ�����Ϊ________________________________________________________��
��3��ͨ��Cװ�õ�����������߽ϳ����ұ߽϶̣�ԭ����________________________
___________________________________________________________________________��
��4��װ��C�ڳ���Ũ��İ��̲��������ڱ����ᣬ��һ�������ǿ�������Ҫ�ɷ�֮һ������0.15molCl2���뷴Ӧʱ�������ɵ������ڱ�״���µ����Ϊ____________��
��5������װ�û�����һ�����Ե�ȱ�ݣ�����Ϊ�Ľ��Ĵ�ʩ��____________________
___________________________________________________________________________��
��1��a e��2�֣�
��2��MnO2+4H++2Cl�� Mn2++Cl2��+2H2O��2�֣���д��Ӧ����ֻ��1�֣�
Mn2++Cl2��+2H2O��2�֣���д��Ӧ����ֻ��1�֣�
��ȥ�����е��Ȼ��⣨1�֣�
��3����ʹ�ܶȽϴ���������ܶȽ�С�İ����ܽϿ�ؾ��Ȼ�ϣ�2�֣�
��4��1.12L��2�֣�
��5����G������һβ������װ�ã�2�֣�
��2��MnO2+4H++2Cl��
 Mn2++Cl2��+2H2O��2�֣���д��Ӧ����ֻ��1�֣�
Mn2++Cl2��+2H2O��2�֣���д��Ӧ����ֻ��1�֣���ȥ�����е��Ȼ��⣨1�֣�
��3����ʹ�ܶȽϴ���������ܶȽ�С�İ����ܽϿ�ؾ��Ȼ�ϣ�2�֣�
��4��1.12L��2�֣�
��5����G������һβ������װ�ã�2�֣�
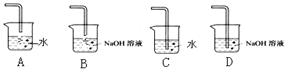
�����������1����ˮ�Ȼ��ƻ��백����Ӧ������ѡ�ã�����������ˮ���ȿ��ԣ���������ˮ���ȿ��ԣ���ʯ�ҿ��ԣ���ˮ����ͭ������ˮ���ȣ����У�
��2��װ��F����ʵ�����������ķ�Ӧ��MnO2+4H++2Cl��
 Mn2++Cl2��+2H2O��Eװ�õ�����Ϊ��ȥ�����е��Ȼ��⡣
Mn2++Cl2��+2H2O��Eװ�õ�����Ϊ��ȥ�����е��Ȼ��⡣��3����ʹ�ܶȽϴ���������ܶȽ�С�İ����ܽϿ�ؾ��Ȼ�ϡ�
��4�����ݷ�Ӧ�ķ���ʽ8NH3+3Cl2=6NH4Cl+N2������0.15molCl2���뷴Ӧʱ�������ɵĵ���Ϊ0.05mol���ڱ�״���µ����Ϊ1.12L��
��5�����������������ж�����Ⱦ��������������β������װ�á�
���������ڽ̲��е�ʵ�飺�������Ʊ�����������ȡװ��Ҫ�������ա����ڱȽ��ۺϵ��е��⡣

��ϰ��ϵ�д�
�����Ŀ

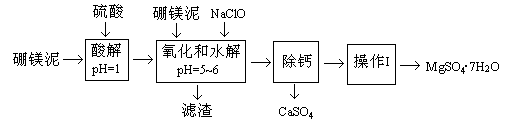

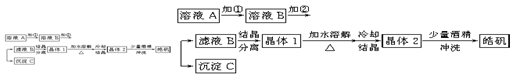
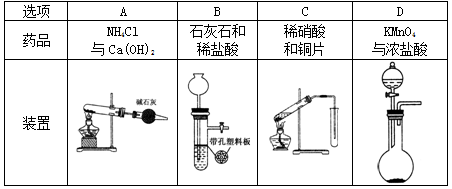
 ��Na2S2O3��Һ����ȷ��ȡNa2S2O3���������
��Na2S2O3��Һ����ȷ��ȡNa2S2O3���������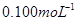 ��Na2S2O3��Һ���еζ����ζ����յ�����Na2S2O3��Һ12��00mL�������KMnO4��Ʒ�Ĵ��ȡ����й����ӷ���ʽΪ��
��Na2S2O3��Һ���еζ����ζ����յ�����Na2S2O3��Һ12��00mL�������KMnO4��Ʒ�Ĵ��ȡ����й����ӷ���ʽΪ��