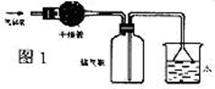
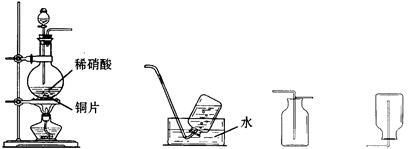
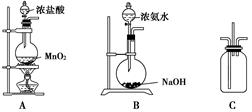
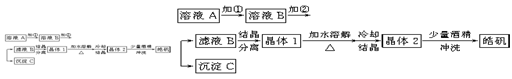
��Ŀ����
ʵ�������ܶ�Ϊ1.18g/mL����������Ϊ36.5%Ũ��������250mL0.1mol/L��������Һ,��ղ���ش��������⣺
(1)�� ����250mL0.1mol/L��������Һ
(2)������ʱ������ȷ�IJ���˳����(��ĸ��ʾ��ÿ����ĸֻ����һ��) ��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳ��ȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��250mL������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
(3)������A�У���ϴ��Һ����������ƿ����Ŀ���� ����Һע������ƿǰ��ָ������£�������Ϊ_____________________��
(4)�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족 ��
��û�н���A���� ����������ˮʱ���������˿̶� ��
������ʱ���ӿ̶��� _______________��
(5)����ʵ������г������������δ�����
������ˮʱ���������˿̶� ��
������ƿ��ת����Һʱ(ʵ�鲽���)������Һ�ε�������ƿ���� ��
(1)�� ����250mL0.1mol/L��������Һ
| Ӧ�����������/mL | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ������������ |
| | | |
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳ��ȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��250mL������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
(3)������A�У���ϴ��Һ����������ƿ����Ŀ���� ����Һע������ƿǰ��ָ������£�������Ϊ_____________________��
(4)�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족 ��
��û�н���A���� ����������ˮʱ���������˿̶� ��
������ʱ���ӿ̶��� _______________��
(5)����ʵ������г������������δ�����
������ˮʱ���������˿̶� ��
������ƿ��ת����Һʱ(ʵ�鲽���)������Һ�ε�������ƿ���� ��
��1��2.1��250����Ͳ���ձ�������������ͷ�ιܡ�
��2��B��C��A��F��E��D
��3����֤����ȫ��ת������ƿ������ƿʢ������Һʱ�������,Ҳ��������ƿը�ѡ�
��4��Ũ��ƫ�ͣ�ƫ�ͣ�ƫ�ߡ�
��5������ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ�����ʵ��ʧ�ܣ�ϴ������ƿ���������ơ�
��2��B��C��A��F��E��D
��3����֤����ȫ��ת������ƿ������ƿʢ������Һʱ�������,Ҳ��������ƿը�ѡ�
��4��Ũ��ƫ�ͣ�ƫ�ͣ�ƫ�ߡ�
��5������ʵ��ʧ�ܣ�ϴ������ƿ���������ƣ�����ʵ��ʧ�ܣ�ϴ������ƿ���������ơ�
�����������1��Ũ�����Ũ��Ϊc=
 ��Ҫ����250mL0.1mol/L��������Һ��Ҫ��Ũ��������ΪV=
��Ҫ����250mL0.1mol/L��������Һ��Ҫ��Ũ��������ΪV= ��Ӧ��ѡ��250mL����ƿ��������Һʱ������Ҫ250mL����ƿ�⣬����Ҫ��Ͳ���ձ�������������ͷ�ιܡ�
��Ӧ��ѡ��250mL����ƿ��������Һʱ������Ҫ250mL����ƿ�⣬����Ҫ��Ͳ���ձ�������������ͷ�ιܡ���2������һ�����ʵ���Ũ����Һ�Ĺ���Ϊ�����㡢��ȡ��ϡ�͡�ת�ơ�ϴ�ӡ����ݡ�ҡ�Ⱥ�װƿ��ǩ������Ӧ����ȷ�IJ���˳��ΪB��C��A��F��E��D��
��3����ϴ��Һȫ��ת������ƿ�е�Ŀ���DZ�֤����ȫ��ת�Ƶ�����ƿ��ע������ƿǰ��Ҫ�ָ�����������Ϊ����ƿʢ������Һʱ������Һ��ȴ�����µ�ʱ���������С����ȷ��Ҳ��������ƿը�ѡ�
��4������c=
 �����û��ϴ�ӣ������ʵ����ʵ���������ֵС������Ũ��ƫ�ͣ���������ˮ���������̶ȣ���Һ�����������ֵ������Ũ��ƫ�ͣ�������ʱ���ӿ̶��ߣ�����Һ�����������ֵС����Ũ��ƫ�ߡ�
�����û��ϴ�ӣ������ʵ����ʵ���������ֵС������Ũ��ƫ�ͣ���������ˮ���������̶ȣ���Һ�����������ֵ������Ũ��ƫ�ͣ�������ʱ���ӿ̶��ߣ�����Һ�����������ֵС����Ũ��ƫ�ߡ���5�������ˮ���������˿̶��ߣ�����Ҫ����Һ������ϴ������ƿ�������ã����ת��ʱ������Һ�������������Ҫ����Һ������ϴ������ƿ�������á�
����������Ƚϻ�������Ҫ����ѧ���Ļ���ʵ�������

��ϰ��ϵ�д�
�����Ŀ
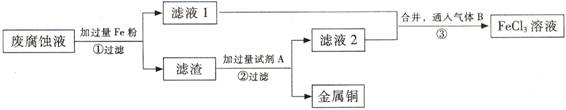 (3) ������з�����Ӧ�����ӷ���ʽ
(3) ������з�����Ӧ�����ӷ���ʽ