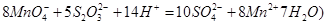��Ŀ����
(12��) ��ͼ��ʵ�������Ʊ��������֤�������ʵ�װ��ͼ

��1������a��������_______________________��
��2��������ͼװ���Ʊ������������������
��Բ����ƿ�ڷ�����Ӧ�����ӷ���ʽΪ_______________________________��
��װ��B�е���ҺΪ____________________���ձ�����Һ������Ϊ________________��
��3������ͼ��ʾԲ����ƿ�ڼ���̼��a�м���Ũ���ᣬ��ʼʵ�飬���Ȳ��������建��ͨ������װ���������ʵ�飺
ʵ��1��֤��SO2����Ư���Ժͻ�ԭ��
ʵ��2��֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ
��B��Ϊ����Ʒ����Һ��C��Ϊ��������KMnO4��Һ,��֤��SO2����Ư���Ե�����Ϊ__________��
��D��Ӧ����������____________������Һ���ƣ���E�м���____________������Һ���ƣ���֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ������Ϊ____________________________��

��1������a��������_______________________��
��2��������ͼװ���Ʊ������������������
��Բ����ƿ�ڷ�����Ӧ�����ӷ���ʽΪ_______________________________��
��װ��B�е���ҺΪ____________________���ձ�����Һ������Ϊ________________��
��3������ͼ��ʾԲ����ƿ�ڼ���̼��a�м���Ũ���ᣬ��ʼʵ�飬���Ȳ��������建��ͨ������װ���������ʵ�飺
ʵ��1��֤��SO2����Ư���Ժͻ�ԭ��
ʵ��2��֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ
��B��Ϊ����Ʒ����Һ��C��Ϊ��������KMnO4��Һ,��֤��SO2����Ư���Ե�����Ϊ__________��
��D��Ӧ����������____________������Һ���ƣ���E�м���____________������Һ���ƣ���֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ������Ϊ____________________________��
��1����Һ©�� ��2����MnO2+4H++2Cl- Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O
�ڱ���ʳ��ˮ ���ն��������,��ֹ��Ⱦ����
��3����B��Ʒ����Һ��ɫ
�����Ը��������Һ����������Һ��D�и��������Һ����ɫ��E����Һ�����
 Mn2++Cl2��+2H2O
Mn2++Cl2��+2H2O�ڱ���ʳ��ˮ ���ն��������,��ֹ��Ⱦ����
��3����B��Ʒ����Һ��ɫ
�����Ը��������Һ����������Һ��D�и��������Һ����ɫ��E����Һ�����
��1������a�Ľṹ���жϣ�a�Ƿ�Һ©����
��2����ʵ������ȡ�������Լ��Ƕ������̺�Ũ���ᣬ����ʽΪ
MnO2+4H++2Cl- Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
������Ũ�����ӷ���������ɵ������к����Ȼ������ʣ�����B�п���ʢ�ű���ʳ��ˮ����ȥ�Ȼ��⣻���������ж���ֱ���ŷŻ���Ⱦ�����������ձ���Ӧ��ʢ������������Һ��ԭ�����ն������������ֹ��Ⱦ������
��3����̼�ڼ��ȵ������£��ܱ�Ũ��������������SO2��CO2�Լ�ˮ���������B��Ʒ����Һ��ɫ�������֤��SO2����Ư���ԡ�
��Ҫ֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ����������ý�ǿ������ȡ����������ʵ�֣��������ɵ�CO2ͨ�뵽��������Һ���ɡ�������SO2Ҳ�ܺ����Ʒ�Ӧ������Ҫ�ȳ�ȥSO2����������SO2�Ļ�ԭ�ԣ�ͨ�����Ը��������Һ��SO2������D�м���������Ը��������Һ����������SO2�Ƿ�����E�м�������ǹ�������Һ����˵�D�и��������Һ����ɫ��E����Һ�����ʱ������֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ��
��2����ʵ������ȡ�������Լ��Ƕ������̺�Ũ���ᣬ����ʽΪ
MnO2+4H++2Cl-
 Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��������Ũ�����ӷ���������ɵ������к����Ȼ������ʣ�����B�п���ʢ�ű���ʳ��ˮ����ȥ�Ȼ��⣻���������ж���ֱ���ŷŻ���Ⱦ�����������ձ���Ӧ��ʢ������������Һ��ԭ�����ն������������ֹ��Ⱦ������
��3����̼�ڼ��ȵ������£��ܱ�Ũ��������������SO2��CO2�Լ�ˮ���������B��Ʒ����Һ��ɫ�������֤��SO2����Ư���ԡ�
��Ҫ֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ����������ý�ǿ������ȡ����������ʵ�֣��������ɵ�CO2ͨ�뵽��������Һ���ɡ�������SO2Ҳ�ܺ����Ʒ�Ӧ������Ҫ�ȳ�ȥSO2����������SO2�Ļ�ԭ�ԣ�ͨ�����Ը��������Һ��SO2������D�м���������Ը��������Һ����������SO2�Ƿ�����E�м�������ǹ�������Һ����˵�D�и��������Һ����ɫ��E����Һ�����ʱ������֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ��

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ
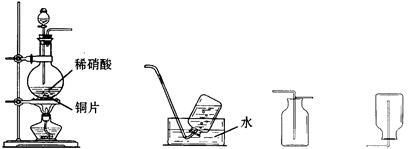
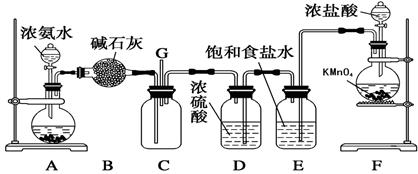
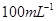 ��Na2S2O3��Һ����ȷ��ȡNa2S2O3���������
��Na2S2O3��Һ����ȷ��ȡNa2S2O3���������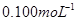 ��Na2S2O3��Һ���еζ����ζ����յ�����Na2S2O3��Һ12��00mL�������KMnO4��Ʒ�Ĵ��ȡ����й����ӷ���ʽΪ��
��Na2S2O3��Һ���еζ����ζ����յ�����Na2S2O3��Һ12��00mL�������KMnO4��Ʒ�Ĵ��ȡ����й����ӷ���ʽΪ��