��Ŀ����
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ������84����Һ��ͨ��ϡ��100��(���֮��)��ʹ�á���ش��������⣺
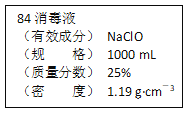
(1)����84����Һ�������ʵ���Ũ��ԼΪ_____mol��L��1��
(2)ȡ����������ĸ�����Һʱ�������������л�����ȡ����Ķ��ٶ��仯����________(����ĸ)��
A����Һ��NaClO�����ʵ��� B����Һ��Ũ��
C����Һ��NaClO��Ħ������ D����Һ���ܶ�
(3)��ͬѧ���ĸ���84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ���ش��������⡣
����ͼ��ʾ�������У���Щ�Dz���Ҫ������������Һ����Ҫ��������_______
����Ҫ����NaClO���������Ϊ_______ g
(4)��84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ��������200 mL 2.3 mol��L��1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ________mol��L��1��
������Ũ��������Ϊ________ mL��
���������Ƶ�ϡ����Ũ��ƫС�������п��ܵ�ԭ���������ȷ����_______��
A������ǰ������ƿ������������ˮ B����ȡŨ����ʱ������Һ��İ�Һ��
C��δ��ȴ������ת��������ƿ���� D������ʱ��������Һ�İ�Һ��
���𰸡�4.0 A ������,��ͷ�ι� 148.8 4.6 25.0 D
��������
(1)����84����Һ�������ʵ���Ũ��ԼΪ![]() mol��L��1��
mol��L��1��
(2)A����Һ��NaClO�����ʵ�������Һ����йأ���Һ���Խ��NaClO�����ʵ���Խ�ࣻ
B����Һ�Ǿ�һ�ȶ��ģ���Ũ�Ⱥ�����ء�
C��NaClO��Ħ��������74.5g/mol������Һ����ء�
D����Һ�Ǿ�һ�ȶ��ģ���Һ���ܶȺ�����ء�
��ѡA��
(3)����480 mL��NaClO��������Ϊ25%������Һ������ʵ����û��480mL������ƿ������Ҫ��500mL������ƿ����500mL����Һ����Ҫ�ȼ������ҪNaClO��������Ȼ������ƽ�Ƴ�NaClO���壬�����ձ��У���������ˮ���ò����������ܽ��ת�Ƶ�����ƿ�У�������ˮϴ�����õ��ձ��Ͳ�����2~3�Σ���ϴ��ҺҲת�Ƶ�����ƿ�У�Ȼ��ֱ��������ƿ�м�����ˮ����̶���1~2cm�ĵط������ý�ͷ�ιܵμ�����ˮ���̶��ߣ�������µߵ�ҡ�ȡ�
����Ҫ���������ձ�����������������ƽ��500mL����ƿ����ͷ�ιܣ����˸������ձ���������ƽ������ƿ�⣬����Ҫ�������ͽ�ͷ�ιܡ�
�ڼ������NaClO�����������500mL��1.19g��cm-3��25%=148.8g��
(4)��2.3 mol��L��1��ϡ�����У�H�������ʵ���Ũ��Ϊ2.3 mol��L��1��2=4.6mol��L��1��
��Ũ��������ʵ���Ũ��Ϊ![]() mol/L������ϡ��ǰ�����ʵ����ʵ������䣬�ɼ�������200 mL 2.3 mol��L��1��ϡ������Ҫ��Ũ��������Ϊ
mol/L������ϡ��ǰ�����ʵ����ʵ������䣬�ɼ�������200 mL 2.3 mol��L��1��ϡ������Ҫ��Ũ��������Ϊ![]() ����25.0 mL��
����25.0 mL��
��A������ǰ������ƿ������������ˮ����Ӱ�����ʵ����ʵ�����Ҳ��Ӱ����Һ����������Զ�������Һ��Ũ����Ӱ�죻
B����ȡŨ����ʱ������Һ��İ�Һ�棬�ᵼ����ȡ��Ũ����ƫ�࣬���Ƶ�ϡ�����Ũ��ƫ�ߣ�
C��δ��ȴ������ת��������ƿ���ݻᵼ����Һ���ƫС�����Ƶ�ϡ�����Ũ��ƫ�ߣ�
D������ʱ��������Һ�İ�Һ��ᵼ����Һ���ƫ�����Ƶ�ϡ�����Ũ��ƫ�͡�
��ѡD��

����Ŀ������̼�Ļ�����������������Ӧ�ù㷺��
��1�����Ȱ���NH2Cl���ĵ���ʽΪ_______________��
��NH2Cl��ˮ��Ӧ����ǿ�����Ե����ʣ�������Ч�������������÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
��2���ý�̿��ԭNO�ķ�ӦΪ��2NO(g)+C(s)![]() N2(g)+CO2(g)�����ݻ���Ϊ1L�ļס��ҡ����������ݺ��£���Ӧ�¶ȷֱ�Ϊ400�桢400�桢T�棩�����зֱ���������Ľ�̿��һ������NO����ø�������n��NO���淴Ӧʱ��t�ı仯������±���ʾ��
N2(g)+CO2(g)�����ݻ���Ϊ1L�ļס��ҡ����������ݺ��£���Ӧ�¶ȷֱ�Ϊ400�桢400�桢T�棩�����зֱ���������Ľ�̿��һ������NO����ø�������n��NO���淴Ӧʱ��t�ı仯������±���ʾ��
t/min | 0 | 40 | 80 | 120 | 160 |
n��NO������������/mol | 2.00 | 1.50 | 1.10 | 0.80 | 0.80 |
n��NO������������/mol | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
n��NO������������/mol | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
�ٸ÷�ӦΪ_______________������ȡ������ȡ�����Ӧ��
����������200min�ﵽƽ��״̬����0��200min����NO��Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(NO)=___________��
��3���ý�̿��ԭNO2�ķ�ӦΪ��2NO2(g)+2C(s)![]() N2(g)+2CO2(g)���ں��������£�1mol NO2������C�����÷�Ӧ�����ƽ��ʱNO2��CO2�����ʵ���Ũ����ƽ����ѹ�Ĺ�ϵ����ͼ��ʾ��
N2(g)+2CO2(g)���ں��������£�1mol NO2������C�����÷�Ӧ�����ƽ��ʱNO2��CO2�����ʵ���Ũ����ƽ����ѹ�Ĺ�ϵ����ͼ��ʾ��
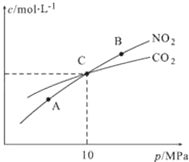
��A��B�����Ũ��ƽ�ⳣ����ϵ��Kc��A��_____Kc��B���������������=������
��A��B��C������NO2��ת������ߵ���__________���A����B����C�����㡣
�ۼ���C��ʱ�÷�Ӧ��ѹǿƽ�ⳣ��Kp��C��=________��Kp����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�����������
��4�������о����֣��ø�Ĥ��ⷨ���Դ�����Ũ����ȩ��ˮ��ԭ����ʹ�ö��Ե缫��⣬��ȩ�ֱ�����������ת��Ϊ�Ҵ������ᣬ�ܷ�ӦΪ��2CH3CHO+H2O![]() CH3CH2OH+CH3COOH��ʵ�����У���һ��Ũ�ȵ���ȩ-Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ���������̣���װ��ʾ��ͼ����ͼ��ʾ��
CH3CH2OH+CH3COOH��ʵ�����У���һ��Ũ�ȵ���ȩ-Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ���������̣���װ��ʾ��ͼ����ͼ��ʾ��
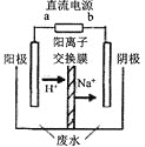
����д���������У������ĵ缫��Ӧʽ��_________________________��
����ʵ�ʴ��������У�����·��I=50Aʱ��10min������ȩ8.8g�������Ч��Ϊ__������������3λ��Ч���֣�ÿ�����ӵĵ���Ϊ1.6��10-19C������Ч��=ʵ�ʷ�Ӧ�������/��·��ͨ��������100%����