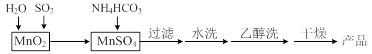��Ŀ����
�������и��������������ݣ��ɷֱ�����䡰���ʵ����������������ʵ����ʵ���
Ũ�ȡ������жϲ���⡣
(1)��NA��ʾ�����ӵ���������ֵ����ij����������ҺV L�к���N��OH��������������Һ��______________________Ϊ__________________��
(2)��֪ij����������Һ��Na����H2O�ĸ���֮��Ϊ1��a������������Һ��________Ϊ________��
(3)��֪��״����1���ˮ���ܽ�500������Ȼ��⣬��������״�����Ȼ��ⱥ����Һ��__________Ϊ________��
(4)��֪��100 mL�Ȼ�����ˮ��Һ�����������գ��ɵõ���ɫ����b g��������ԭ�Ȼ�����Һ��________Ϊ________��
(1)���ʵ����ʵ���Ũ��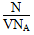 mol/L
mol/L
(2)���ʵ���������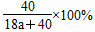
(3)���ʵ�����������44.9%
(4)���ʵ����ʵ���Ũ��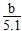 mol/L
mol/L
����

��ϰ��ϵ�д�
�����Ŀ
��MgCl2��Cl2��Ca(ClO)2��HClO2��(����)��Cl2O7�������У�������Ӧ��������ʵĻ�ѧʽ��
| A��Cl2O3 | B��KClO4 | C��KClO3 | D��HClO |
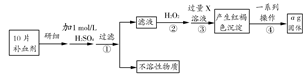

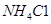 ��Һ��
��Һ�� ��Һ�����ԣ�������ƽ��ԭ���ͱ�Ҫ�����ֽ�������C������3mol/L��
��Һ�����ԣ�������ƽ��ԭ���ͱ�Ҫ�����ֽ�������C������3mol/L��