��Ŀ����
ú�������Ǹ�Ч����������ú̿����Ҫ;��֮һ��
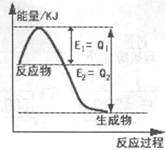
(1)��250C 101kPaʱ��H2��O2��������1mol H2O(g)�ų�241.8kJ�����������Ȼ�ѧ����ʽΪ
___________
��֪: ��C(s)��O2(g)�TCO2(g) ��H����393.5kJ/mol
��CO(g)��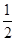 O2(g)�TCO2(g) ��H����283.0kJ/mol
O2(g)�TCO2(g) ��H����283.0kJ/mol
��̿��ˮ������Ӧ�ǽ�����ú��Ϊ����ȼ�ϵķ�����C(s)��H2O(g)�TCO(g)��H2(g) ��H=____kJ/mol
(2) CO������H2O(g)��һ��������Ӧ: CO(g)��H2O(g) CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)��
CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)��
(3) ��ҵ�ϴ�ú������Ļ�����з����H2�����а��ĺϳɣ���֪��Ӧ��ӦN2(g)��3H2(g 2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
��N2��ƽ����Ӧ����v1(N2)��vII(N2)��vIII(N2)�Ӵ�С���д���Ϊ________��
���ɵ�һ��ƽ��ڶ���ƽ�⣬ƽ���ƶ��ķ��� ��________����ȡ�Ĵ�ʩ��________��
�۱Ƚϵ�II�η�Ӧ�¶�(T2)�͵�III�η�Ӧ�ٶȣ�T3)�ĸߵͣ�T2________T3�����=��<���жϵ�������________________��
��14�֣���1��H2(g)+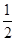 O2(g)�TlH2O(g) ��H����241.8kJ/mol ��2�֣�����131.3kJ ��1�֣�
O2(g)�TlH2O(g) ��H����241.8kJ/mol ��2�֣�����131.3kJ ��1�֣�
��2��66.7% ��2�֣� ��3����v1(N2)��vII(N2)��vIII(N2)��3�֣�
��������Ӧ���� �ӷ�Ӧ��ϵ���Ƴ�����NH3��3�֣�
�ۣ� �˷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ�����ƶ� ��3�֣�
���������������1����25�桢101kPaʱ��H2��O2��������1molH2O(g)�ų�241.8kJ���������������Ȼ�ѧ����ʽΪH2(g)+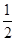 O2(g)�TlH2O(g) ��H����241.8kJ/mol��
O2(g)�TlH2O(g) ��H����241.8kJ/mol��
��֪��C(s)��O2(g)�TCO2(g) ��H����393.5kJ/mol
��CO(g)��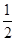 O2(g)�TCO2(g) ��H����283.0kJ/mol
O2(g)�TCO2(g) ��H����283.0kJ/mol
��H2(g)+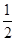 O2(g)�TlH2O(g) ��H����241.8kJ/mol
O2(g)�TlH2O(g) ��H����241.8kJ/mol
���Ը��ݸ�˹���ɣ��٣��ۣ��ڼ��õ�C(s)��H2O(g)�TCO(g)��H2(g)����Ӧ�ȡ�H����393.5kJ/mol��241.8kJ/mol��283.0kJ/mol����131.3kJ/mol��
��2�� CO(g)��H2O(g) CO2(g)��H2(g)
CO2(g)��H2(g)
��ʼ����mol�� 0.1 0.2 0 0
ת������mol�� x x x x
ƽ������mol�� 0.1��x 0.2��x x x
���ڷ�Ӧǰ�������������䣬���������ʵ�������Ũ�ȼ���ƽ�ⳣ��
�� ��1.0
��1.0
���x��
����ƽ��ʱ��������CO��ת������ ��100%��66.7%
��100%��66.7%
��3���ٸ���ͼ���֪�����߱�ʾ������Ũ�ȱ仯����v1(N2)����2mol/L��1mol/L����20min��0.05mol/��L?min����vII(N2)����1mol/L��0.62mol/L����15min��0.0253mol/��L?min����vIII(N2)����0.62mol/L��0.5mol/L����10min��0.012mol/��L?min������N2��ƽ����Ӧ����v1(N2)��vII(N2)��vIII(N2).
�ڸ���ͼ���֪���ڢ�ΰ����Ǵ�0��ʼ�ģ�˲�䷴Ӧ�ﵪ��������Ũ�Ȳ��䣬��˿���ȷ����һ��ƽ������ϵ���Ƴ��˰�����������������Ũ�ȣ�ƽ�������ƶ���
�۵ڢ�εĿ�ʼ��ڢ�ε�ƽ������ʵ�������ȣ����ݰ����������������٣������������ӿ��ж�ƽ���������ƶ��ġ�����ƽ�ʼʱŨ��ȷ����ƽ���ƶ�����������Ũ�ȵı仯����ģ�������Ŀ��������������������䣬��ı�ѹǿҲ�����ܣ����һ��Ϊ�¶ȵ�Ӱ�졣�˷�Ӧ����Ϊ���ȷ�Ӧ�������Ʋ�Ϊ�����¶ȣ���˴ﵽƽ����¶�һ���ȵڢ��ƽ��ʱ���¶ȵͣ���T2��T3��
���㣺�����Ȼ�ѧ����ʽ����д����˹���ɵ�Ӧ�ã���Ӧ���ʺ�ƽ�ⳣ���ļ����Լ���������Է�Ӧ���ʺ�ƽ��״̬��Ӱ���

 ��У����ϵ�д�
��У����ϵ�д���������;�㷺����Ҫ��������Ӳ�ʻ����µĺϽ��Լ����ݵĵ�˿�������£����ܱ���������H2��ԭWO3�ɵõ������٣����ܷ�ӦΪ��
WO3 (s) + 3H2 (g) W (s) + 3H2O (g)
W (s) + 3H2O (g)
��ش��������⣺
��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ___________________________��
��ij�¶��·�Ӧ��ƽ��ʱ��H2��ˮ�����������Ϊ2:3����H2��ƽ��ת����Ϊ_____________________�����¶ȵ����ߣ�H2��ˮ����������ȼ�С����÷�ӦΪ��Ӧ_____________________������ȡ����ȡ�����
�������ܷ�Ӧ���̴��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���±���ʾ��
| �¶� | 25�� ~ 550�� ~ 600�� ~ 700�� |
| ��Ҫ�ɷ� | WO3 W2O5 WO2 W |
��һ�η�Ӧ�Ļ�ѧ����ʽΪ___________________________��580��ʱ���������ʵ���Ҫ�ɷ�Ϊ________������WO3��ȫת��ΪW��������������H2���ʵ���֮��Ϊ____________________________________��
�� ��֪���¶ȹ���ʱ��WO2 (s)ת��ΪWO2 (g)��
WO2 (s) + 2H2 (g)
 W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1
W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1 WO2 (g) + 2H2(g)
 W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1
W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1 ��WO2 (s)
 WO2 (g) �Ħ�H �� ______________________��
WO2 (g) �Ħ�H �� ______________________������˿�ƹ��е�W��ʹ�ù����л����ӷ���ʹ��˿��ϸ������I2���ӳ��ƹܵ�ʹ���������乤��ԭ��Ϊ��W (s) +2I2 (g)
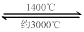 WI4 (g)������˵����ȷ����____________��
WI4 (g)������˵����ȷ����____________��a���ƹ��ڵ�I2��ѭ��ʹ��
b��WI4�ڵ�˿�Ϸֽ⣬������W�ֳ����ڵ�˿��
c��WI4�ڵƹܱ��Ϸֽ⣬ʹ�ƹܵ������ӳ�
d���¶�����ʱ��WI4�ķֽ����ʼӿ죬W��I2�Ļ������ʼ���
����(H2NCONH2)��һ�ַdz���Ҫ�ĸߵ����ʣ��ڹ�ũҵ���������ŷdz���Ҫ�ĵ�λ��
��1����ҵ�Ϻϳ����صķ�Ӧ���£�
2NH3(l)+CO2(g) H2O(l)+H2NCONH2(l) ��H=-103��7 kJ��mol-1
H2O(l)+H2NCONH2(l) ��H=-103��7 kJ��mol-1
���д�ʩ��������������ص��������ʵ���
| A�����ø��� |
| B�����ø�ѹ |
| C��Ѱ�Ҹ���Ч�Ĵ��� |
| D����С��ϵ��CO2Ũ�� |
��һ����2NH3(l)+CO2(g)
 H2NCOONH4(���������)(l) ��H1
H2NCOONH4(���������)(l) ��H1�ڶ�����H2NCOONH4(l)
 H2O(l)+H2NCONH2(l) ��H2��
H2O(l)+H2NCONH2(l) ��H2��ijʵ��С��ģ�ҵ�Ϻϳ����ص���������һ���Ϊ0��5 L�ܱ�������Ͷ��4 mol����l mol������̼��ʵ���÷�Ӧ�и������ʱ��ı仯����ͼI��ʾ��

����֪�ܷ�Ӧ�Ŀ���������һ����������ϳ������ܷ�Ӧ�Ŀ����ɵ� ����Ӧ�������ܷ�Ӧ���е� minʱ����ƽ�⡣
�ڵڶ�����Ӧ��ƽ�ⳣ��K���¶ȵı仯����ͼII��ʾ�����H2 0(�>�� ��<�� �� ��=��)
��3�����¶�70-95��ʱ����ҵβ���е�NO��NO2������������Һ���գ�����ת��ΪN2
��������NO��NO2���ߵ����ʵ�����Ӧ����ѧ����ʽΪ
����֪��N2(g)+O2(g)=2NO(g)����H=180��6 kJ��mol-1
N2(g)+3H2(g)=2NH3(g) ��H=-92��4 kJ��mol-1
2H2(g)+O2(g)=2H2O(g) ��H=-483��6 kJ��mol-1
��4NO(g)+4NH3(g)+O2(g)=4N2(g)+6H2O(g) ��H= kJ��mol-1
��4������ȼ�ϵ�ؽṹ����ͼIII��ʾ���乤��ʱ�����缫��Ӧʽ
�ɱ�ʾΪ ��

 2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ�� 
 2SO3(g)�Ļ����ϵ�У�SO3�İٷֺ������¶ȵĹ�ϵ����ͼ(�������κ�һ�㶼��ʾƽ��״̬����
2SO3(g)�Ļ����ϵ�У�SO3�İٷֺ������¶ȵĹ�ϵ����ͼ(�������κ�һ�㶼��ʾƽ��״̬����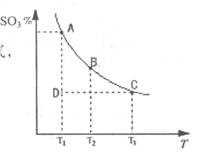

 2S(s) + 2H2O(g) ��H= ��442.38 kJ/mol ��
2S(s) + 2H2O(g) ��H= ��442.38 kJ/mol ��