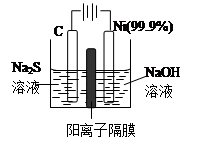
��Ŀ����
��14�֣���������Ȼ���еIJ���ѭ����ϵ���¡�
��1��H2S�ڿ����п���ȼ�ա�
��֪�� 2H2S(g) + O2(g)  2S(s) + 2H2O(g) ��H= ��442.38 kJ/mol ��
2S(s) + 2H2O(g) ��H= ��442.38 kJ/mol ��
S(s) + O2(g)  SO2(g) ��H=��297.04 kJ/mol ��
SO2(g) ��H=��297.04 kJ/mol ��
H2S(g)��O2(g)��Ӧ����SO2(g)��H2O(g)���Ȼ�ѧ����ʽ�� ��
��2��SO2�Ǵ�����Ⱦ���ˮ�������õ�����SO2����������������¡�
�� SO2���ں�ˮ����H2SO3��H2SO3���ջ�����SO32��������뷽��ʽ�� ��
�� SO32�����Ա���ˮ�е��ܽ�������ΪSO42������ˮ��pH�� ������ߡ� �������䡱���͡�����
�� Ϊ������ˮ��pH���ɼ������ʵĺ�ˮ��ʹ���е�HCO3�����뷴Ӧ���䷴Ӧ�����ӷ���ʽ�� ��
�� ��������Ӧ��ͬʱ��Ҫ���������������ԭ���� ��
��3����Ȼ��ر���ԭ��ͭ�����ᆳ�������������ú���CuSO4��Һ���������������������ܵ�ZnS������ת��Ϊͭ����CuS�����û�ѧ�����ʾ��ZnSת��ΪCuS�Ĺ��̣� ��
��4��SO2��O2��H2SO4��Һ�п��Թ���ԭ��أ��为����Ӧʽ�� ��
��14�֣�ÿ��2�֣�
��1��2H2S(g)+3O2(g)  2SO2(g)+2H2O(g) ��H= ��1036.46 kJ/mol ������ʽ�ͼ����1�֣�
2SO2(g)+2H2O(g) ��H= ��1036.46 kJ/mol ������ʽ�ͼ����1�֣�
��2���� H2SO3 HSO3��+ H+ HSO3��
HSO3��+ H+ HSO3�� SO3 2��+ H+ ��ÿ��1�֣�
SO3 2��+ H+ ��ÿ��1�֣�
�� ����
�� HCO3 ��+ H+  CO2 ��+ H2O������1�֣���ƽ1�֣�
CO2 ��+ H2O������1�֣���ƽ1�֣�
���������ˮ���ܽ�������SO32-������ΪSO42-��������ƽ��H2SO3 HSO3��+ H+ HSO3��
HSO3��+ H+ HSO3�� SO3 2��+ H+ �����ƶ���1�֣�����߶��������ת���ʣ�ͬʱ�ӿ췴Ӧ���ʵ����ã�1�֣���
SO3 2��+ H+ �����ƶ���1�֣�����߶��������ת���ʣ�ͬʱ�ӿ췴Ӧ���ʵ����ã�1�֣���
��3��
��4��������SO2 - 2e��+ 2H2O  SO4 2��+ 4H+������1�֣���ƽ1�֣�
SO4 2��+ 4H+������1�֣���ƽ1�֣�
���������������1�����ݸ�˹���ɵ�Ŀ�귽��ʽ=��+2���ڣ�����H2S(g)��O2(g)��Ӧ����SO2(g)��H2O(g)���Ȼ�ѧ����ʽ��2H2S(g)+3O2(g) 2SO2(g)+2H2O(g) ��H= ��1036.46 kJ/mol
2SO2(g)+2H2O(g) ��H= ��1036.46 kJ/mol
��2���������������ᣬ�ֲ������SO3 2�������뷽��ʽ��H2SO3 HSO3��+ H+ HSO3��
HSO3��+ H+ HSO3�� SO3 2��+ H+
SO3 2��+ H+
��SO32�����Ա���ˮ�е��ܽ�������ΪSO42����������ǿ�ᣬ��Һ�е�������Ũ������pH���ͣ�
�����ʵĺ�ˮ�����ԣ���HCO3����Ӧ���ɶ�����̼��ˮ�����ӷ���ʽΪHCO3 ��+ H+  CO2 ��+ H2O
CO2 ��+ H2O
�ܺ�ˮ�������õ�����SO2���������������������������ߺ�ˮ�еĺ���������SO32-������ΪSO42-��������ƽ��H2SO3 HSO3��+ H+ HSO3��
HSO3��+ H+ HSO3�� SO3 2��+ H+ �����ƶ�����߶��������ת���ʣ�ͬʱ�ӿ췴Ӧ���ʵ����ã�
SO3 2��+ H+ �����ƶ�����߶��������ת���ʣ�ͬʱ�ӿ췴Ӧ���ʵ����ã�
��3��CuS��ZnS�����ܣ���п����ZnS(s)  Zn2+(aq)+S2��(aq)ƽ�⣬����������ͭ��Һʱ��S2����Cu2+�������CuS������ʹ�ܽ�ƽ�������ƶ�������ZnSȫ��ת��ΪCuS���û�ѧ�����ʾΪ
Zn2+(aq)+S2��(aq)ƽ�⣬����������ͭ��Һʱ��S2����Cu2+�������CuS������ʹ�ܽ�ƽ�������ƶ�������ZnSȫ��ת��ΪCuS���û�ѧ�����ʾΪ
��4����������������Ӧ��Ԫ�ػ��ϼ����ߣ������Ƕ��������ڸ���������Ӧ��������������ӣ��缫��ӦʽΪSO2 - 2e��+ 2H2O  SO4 2��+ 4H+
SO4 2��+ 4H+
���㣺�����˹���ɵ�Ӧ�ã����뷽��ʽ����д���ܽ�ƽ����ƶ����绯ѧԭ����Ӧ��

 �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д��о����仯�������������Ҫ���塣
��1��Cu2S�ڸ��������·������·�Ӧ��
2Cu2S(s)+3O2(g)=2Cu2O(s)+2SO2(g) �SH=��773kJ/mol
���÷�Ӧ��1.2mol����ת��ʱ,��Ӧ�ͷų�������Ϊ kJ��
��2�����Ṥҵ�������漰��Ӧ��2SO2(g)+O2(g) 2SO3(g)��SO2��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
2SO3(g)��SO2��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
��ѹǿ��P1 P2�����������=����<������
��ƽ�ⳣ����A�� B�㣨���������=����<������
��200���£���һ������SO2��O2�������������ܱ������У���10min���������и����ʵ����ʵ���Ũ�����±���ʾ:
| ���� | SO2 | O2 | SO3 |
| Ũ�ȣ�mol/L�� | 0.4 | 1.2 | 1.6 |
��˵���÷�Ӧ�ﵽ��ѧƽ��״̬���� ��
a��SO2��O2������ȱ��ֲ���
b����ϵ��ѹǿ���ֲ���
c�����������ܶȱ��ֲ���
d��SO2��SO3���ʵ���֮�ͱ��ֲ���
����������Ӧ��0~10min�ڣ���(O2)= ��
��3��һ���¶��£���ˮ����SO2����ʱ����Һ��ˮ�ĵ���ƽ�� �ƶ�����������ҡ������������õ�pH=3��H2SO3��Һ���Լ�����Һ��
 ������֪���¶���H2SO3�ĵ��볣����Ka1=1.0��10-2 mol/L��Ka2=6.0��10-3 mol/L��
������֪���¶���H2SO3�ĵ��볣����Ka1=1.0��10-2 mol/L��Ka2=6.0��10-3 mol/L�� ������������Ȼ����Ϊԭ�Ϻϳɼ״������ⱻһһ���ˣ�����شٽ��˼״���ѧ�ķ�չ��
��1����̿��ˮ�����ķ�Ӧ���ƣ�����Ȼ��Ϊԭ��Ҳ�����Ƶ�CO��H2���÷�Ӧ�Ļ�ѧ����ʽΪ_________��
��2���ϳɼ״���һ�ַ�������CO��H2Ϊԭ�ϣ��������仯��ͼ��ʾ��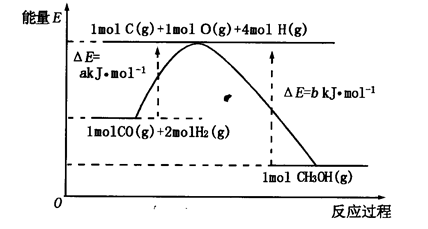
��ͼ��֪���ϳɼ״����Ȼ�ѧ����ʽΪ________________________________________��
��3����CO2Ϊԭ��Ҳ���Ժϳɼ״����䷴Ӧԭ��Ϊ��CO2(g)+3H2(g) CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
����lL���ܱ������У�����1molCO2��3molH2����500���·�����Ӧ�����CO2(g)��CH3OH(g)��Ũ����ʱ�ʱ仯��ͼ��ʾ��
������˵����ȷ����_________________(����ĸ)��
| A��3minʱ��Ӧ�ﵽƽ�� |
| B��0��10minʱ��H2��ʾ�ķ�Ӧ����Ϊ0��225mol��-1��min-1 |
| C��CO2��ƽ��ת����Ϊ25�� |
D�����¶�ʱ��ѧƽ�ⳣ��Ϊ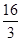 ��mol/L����2 ��mol/L����2 |
| ���� | ����1 | ����2 | ����3 |
| ��Ӧ��Ͷ������ʼ̬�� | 1molCO2��3molH2 | 0.5molCO2��1.5molH2 | 1molCH3OH��1molH2O |
| CH3OH��ƽ��Ũ��/mol?L-1 | c1 | c2 | c3 |
| ƽ��ʱ��ϵѹǿ/Pa | p1 | p2 | p3 |
�����и����Ĵ�С��ϵΪc1___________c3��p2_________p3(����ڡ��������ڡ���С�ڡ�)��
��4�����������״�ȼ�ϵ�ؼ���������µ�ͻ�ƣ���ͼ��ʾΪ�״�ȼ�ϵ�ص�װ��ʾ��ͼ����ع���ʱ���ֱ��b��c����CH3OH��O2���ش��������⣺

�ٴ�d���ų���������___________����Һ�е���������缫__________(�M����N��)��
�ڵ缫M�Ϸ����ĵ缫��ӦʽΪ__________________________��
Ŀǰ��ҵ�ϳɰ���ԭ���ǣ�N2(g)+3H2(g) 2NH3(g) ��H=��93.0kJ /mol�����ݱ�����һ�������£�2N2(g)+6H2O(l)
2NH3(g) ��H=��93.0kJ /mol�����ݱ�����һ�������£�2N2(g)+6H2O(l) 4NH3(g)+3O2(g) ��H=" +1530.0kJ" /mol��
4NH3(g)+3O2(g) ��H=" +1530.0kJ" /mol��
��1��������ȼ���ȡ�H=_______________kJ/mol��
��2���ں��º�ѹװ���н��й�ҵ�ϳɰ���Ӧ������˵����ȷ���� ��
| A������������ٱ仯������ƽ�� |
| B�������ܶȲ��ٱ仯����δƽ�� |
| C��ƽ�����װ����ͨ��һ����Ar��ѹǿ���䣬ƽ�ⲻ�ƶ� |
| D��ƽ���ѹ��װ�ã����ɸ���NH3 |
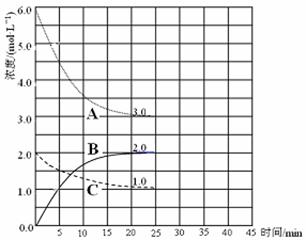
�� ��ʾN2Ũ�ȱ仯�������� ��
�� ǰ25 min �ڣ���H2Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ������ ��
�� ��25 minĩ�պ�ƽ�⣬��ƽ�ⳣ��K = ��
��4���ڵ�25 min ĩ�����������������䣬�����¶ȣ��ڵ�35 minĩ�ٴ�ƽ�⡣ƽ���ƶ�������H2Ũ�ȱ仯��1.5 mol��L��1����ͼ�л�����25 min �� 40 min NH3Ũ�ȱ仯���ߡ�
��5����֪�����£�NH4+ ��ˮ�ⳣ��Ϊ1.0��10��9����0.1mol/L NH4Cl��ҺpH= ��������NH4+ˮ���NH4+Ũ�ȵ�Ӱ�죩
ij�¶��£���2L������3�����ʼ���з�Ӧ�� X��Y��Z�����ʵ�����ʱ��ı仯������ͼ����������������� 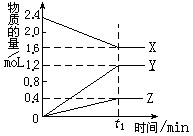
A���÷�Ӧ�Ļ�ѧ����ʽ��2X 3Y+Z 3Y+Z |
| B����t1minʱ���÷�Ӧ�ﵽ��ƽ��״̬ |
| C��t1minʱ��X��Y��Z�ķ�Ӧ������� |
| D�����÷�Ӧ�Ѵﵽƽ��״̬ʱ��ÿ����1molZ��ͬʱ����2molX |
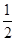 O2(g)�TCO2(g) ��H����283.0kJ/mol
O2(g)�TCO2(g) ��H����283.0kJ/mol CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)��
CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)�� 2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
 2SO3(g)��ƽ�ⳣ��Ϊ ��
2SO3(g)��ƽ�ⳣ��Ϊ ��