ЬтФПФкШн
ЁОЬтФПЁПИпЗжзгHЪЧвЛжжГЩФЄСМКУЕФЪїжЌЃЌЦфКЯГЩТЗЯпШчЯТЃК

вбжЊЃКЂйAЕФЯрЖдЗжзгжЪСПЮЊ58ЃЌбѕдЊЫижЪСПЗжЪ§ЮЊ0.276ЃЌКЫДХЙВеёЧтЦзЯдЪОжЛгавЛзщЗхЃЛ
Ђк ![]()
(1)AЕФНсЙЙМђЪНЮЊ ____________________________ЃЌGжаЙйФмЭХУћГЦЮЊ_________________
(2)гЩBЩњГЩCЕФЛЏбЇЗНГЬЪНЮЊ___________________________________________________ЁЃ
(3)DЕФЯЕЭГУќУћЮЊ_________________ЁЃ
(4)ЛЏКЯЮяEЕФЗаЕу ______ (бЁЬюЁА>ЁБЃЌЁА<ЁБЛђепЁА=ЁБ)2-МзЛљБћЭщЁЃ
(5)FгыаТжЦCu(OH)2аќзЧвКЗДгІЕФЛЏбЇЗНГЬЪНЮЊ____________________________________ЁЃ
(6)HЕФНсЙЙМђЪНЮЊ________________________________________ЁЃ
(7) ЕФЗћКЯЯТСаЬѕМўЕФЭЌЗжвьЙЙЬхга____жж
ЕФЗћКЯЯТСаЬѕМўЕФЭЌЗжвьЙЙЬхга____жж
ЂйЗжзгжаЮоЛЗзДНсЙЙЧвЮожЇСД ЂкКЫДХЙВеёЧтЦзгаСНзщЗхЃЌЗхУцЛ§жЎБШЮЊ3:2ЦфжаЙйФмЭХФмгыH2ЗЂЩњМгГЩЗДгІЕФгаЛњЮяНсЙЙМђЪНЮЊ_____________(ШЮаДвЛжж)ЁЃ
ЁОД№АИЁП![]() єШЛљ
єШЛљ ![]() 2-МзЛљ-1,2-ЖўфхБћЭщ >
2-МзЛљ-1,2-ЖўфхБћЭщ > 
 6
6 

ЁОНтЮіЁП
AЕФЯрЖдЗжзгжЪСПЮЊ58ЃЌбѕдЊЫижЪСПЗжЪ§ЮЊ0.276ЃЌдђAЗжзгжабѕдзгЪ§ФПЮЊ![]() =1ЃЌЗжзгжаCЁЂHдзгзмЯрЖддзгжЪСПЮЊ58-16=42ЃЌдђЗжзгжазюДѓЬМдзгЪ§ФПЮЊ
=1ЃЌЗжзгжаCЁЂHдзгзмЯрЖддзгжЪСПЮЊ58-16=42ЃЌдђЗжзгжазюДѓЬМдзгЪ§ФПЮЊ![]() =3Ё6ЃЌЙЪAЕФЗжзгЪНЮЊC3H6OЃЌЦфКЫДХЙВеёЧтЦзЯдЪОжЛгавЛзщЗхЃЌЧвЗЂЩњаХЯЂжаМгГЩЗДгІЩњГЩBЃЌЙЪAЮЊ
=3Ё6ЃЌЙЪAЕФЗжзгЪНЮЊC3H6OЃЌЦфКЫДХЙВеёЧтЦзЯдЪОжЛгавЛзщЗхЃЌЧвЗЂЩњаХЯЂжаМгГЩЗДгІЩњГЩBЃЌЙЪAЮЊ![]() ЃЌBЮЊ
ЃЌBЮЊ![]() ЃЌBдкХЈСђЫсДпЛЏЯТЗЂЩњЯћШЅЗДгІЩњГЩCЮЊ
ЃЌBдкХЈСђЫсДпЛЏЯТЗЂЩњЯћШЅЗДгІЩњГЩCЮЊ![]() ЃЌCгыфхЗЂЩњМгГЩЗДгІЩњГЩDЮЊCH3CBr(CH3)CH2BrЃЌDдкЧтбѕЛЏФЦЕФЫЎШмвКжаМгШШЗЂЩњЫЎНтЗДгІЩњГЩEЮЊCH3C(CH3)OHCH2OHЃЛ
ЃЌCгыфхЗЂЩњМгГЩЗДгІЩњГЩDЮЊCH3CBr(CH3)CH2BrЃЌDдкЧтбѕЛЏФЦЕФЫЎШмвКжаМгШШЗЂЩњЫЎНтЗДгІЩњГЩEЮЊCH3C(CH3)OHCH2OHЃЛ дкДпЛЏМСзїгУЯТгыбѕЦјЗЂЩњбѕЛЏЗДгІЩњГЩFЮЊ
дкДпЛЏМСзїгУЯТгыбѕЦјЗЂЩњбѕЛЏЗДгІЩњГЩFЮЊ ЃЌFгыаТжЦЕФЧтбѕЛЏЭШмвКЗЂЩњЗДгІКѓЫсЛЏЕУЕНGЮЊ
ЃЌFгыаТжЦЕФЧтбѕЛЏЭШмвКЗЂЩњЗДгІКѓЫсЛЏЕУЕНGЮЊ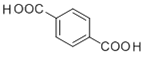 ЃЌEгыGдквЛЖЈЬѕМўЯТЗЂЩњЫѕОлЗДгІЩњГЩHЮЊ
ЃЌEгыGдквЛЖЈЬѕМўЯТЗЂЩњЫѕОлЗДгІЩњГЩHЮЊ ЁЃ
ЁЃ
(1)AЕФНсЙЙМђЪНЮЊ![]() ЃЌGЮЊ
ЃЌGЮЊ ЃЌЙйФмЭХУћГЦЮЊєШЛљЃЛ
ЃЌЙйФмЭХУћГЦЮЊєШЛљЃЛ
(2)гЩBЩњГЩCЮЊ![]() дкХЈСђЫсзїгУЯТЗЂЩњЯћШЅЗДгІЩњГЩ
дкХЈСђЫсзїгУЯТЗЂЩњЯћШЅЗДгІЩњГЩ![]() КЭЫЎЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊ
КЭЫЎЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊ![]() ЃЛ
ЃЛ
(3)DЮЊCH3CBr(CH3)CH2BrЃЌЯЕЭГУќУћЮЊ2-МзЛљ-1,2-ЖўфхБћЭщЃЛ
(4)ЛЏКЯЮяEЮЊCH3C(CH3)OHCH2OHЃЌОпгаЯрЭЌЬМдзгЪ§МАНсЙЙЯрЫЦЕФЭщЬўЗаЕуЕЭгкДМЃЌЙЪEЕФЗаЕу>2-МзЛљБћЭщЃЛ
(5)FЮЊ ЃЌгыаТжЦCu(OH)2аќзЧвКЗДгІЕФЛЏбЇЗНГЬЪНЮЊ
ЃЌгыаТжЦCu(OH)2аќзЧвКЗДгІЕФЛЏбЇЗНГЬЪНЮЊ ЃЛ
ЃЛ
(6)HЕФНсЙЙМђЪНЮЊ ЃЛ
ЃЛ
(7) ЕФЭЌЗжвьЙЙЬхЃЌЗћКЯЂйЗжзгжаЮоЛЗзДНсЙЙЧвЮожЇСДЃЌдђВЛБЅКЭЖШЮЊ4ЃЌЂкКЫДХЙВеёЧтЦзгаСНзщЗхЃЌЗхУцЛ§жЎБШЮЊ3:2ЃЌдђИпЖШЖдГЦжЛгаСНжжЛЏбЇЛЗОГЯТЕФЧтЃЌЗћКЯЬѕМўЕФЭЌЗжвьЙЙЬхга
ЕФЭЌЗжвьЙЙЬхЃЌЗћКЯЂйЗжзгжаЮоЛЗзДНсЙЙЧвЮожЇСДЃЌдђВЛБЅКЭЖШЮЊ4ЃЌЂкКЫДХЙВеёЧтЦзгаСНзщЗхЃЌЗхУцЛ§жЎБШЮЊ3:2ЃЌдђИпЖШЖдГЦжЛгаСНжжЛЏбЇЛЗОГЯТЕФЧтЃЌЗћКЯЬѕМўЕФЭЌЗжвьЙЙЬхга ЁЂ
ЁЂ ЁЂCH3C
ЁЂCH3C![]() C-O-CH2CH2-O-C
C-O-CH2CH2-O-C![]() CCH3ЁЂCH3-O-C
CCH3ЁЂCH3-O-C![]() CCH2CH2C
CCH2CH2C![]() C-O-CH3ЁЂCH3OCH2C
C-O-CH3ЁЂCH3OCH2C![]() C-C
C-C![]() CCH2OCH3ЁЂCH3CH2O-C
CCH2OCH3ЁЂCH3CH2O-C![]() C-C
C-C![]() C-OCH2CH3ЙВ6жжЃЛЦфжаЙйФмЭХФмгыH2ЗЂЩњМгГЩЗДгІЕФгаЛњЮяНсЙЙМђЪНЮЊ
C-OCH2CH3ЙВ6жжЃЛЦфжаЙйФмЭХФмгыH2ЗЂЩњМгГЩЗДгІЕФгаЛњЮяНсЙЙМђЪНЮЊ ЁЂ
ЁЂ ЁЃ
ЁЃ

 гІгУЬтзївЕБОЯЕСаД№АИ
гІгУЬтзївЕБОЯЕСаД№АИЁОЬтФПЁПФГЪЕбщаЁзщдкГЃЮТЯТНјааЕчНтБЅКЭCa(OH)2ШмвКЕФЪЕбщЃЌЪЕбщзАжУгыЯжЯѓМћЯТБэЁЃ
ађКХ | I | II |
зАжУ |
|
|
ЯжЯѓ | СНМЋОљВњЩњДѓСПЦјХнЃЌbМЋБШaМЋЖрЃЛaМЋШмвКж№НЅВњЩњАзЩЋЛызЧЃЌИУАзЩЋЛызЧМгШыбЮЫсгаЦјХнВњЩњ | СНМЋОљВњЩњДѓСПЦјХнЃЌdМЋБШcМЋЖрЃЛcМЋБэУцВњЩњЩйСПКкЩЋЙЬЬхЃЛcМЋШмвКЮДМћАзЩЋЛызЧ |
ЯТСаЙигкЪЕбщЯжЯѓЕФНтЪЭгыЭЦТлЃЌе§ШЗЕФЪЧЃЈ ЃЉ
A. aМЋШмвКВњЩњАзЩЋЛызЧЕФжївЊдвђЪЧЕчНтЙ§ГЬЯћКФЫЎЃЌЮіГіCa(OH)2ЙЬЬх
B. bМЋВњЩњЦјХнЃК4OHЃ Ѓ4eЃ === O2Ёќ ЃЋ2H2O
C. cМЋБэУцБфКкЃКCu Ѓ2eЃ ЃЋ2OHЃ === CuO ЃЋH2O
D. dМЋЕчМЋЗДгІЕФЗЂЩњЃЌвжжЦСЫЫЎЕФЕчРы







