��Ŀ����
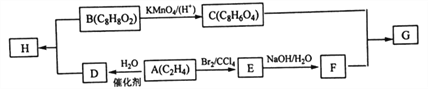
����Ŀ��A��B��C��D��E���ֻ������������ͬ��һ��Ԫ��X��C��D��ˮ��Һ�ֱ���AgNO3��Һ���ʱ�����е�Ԫ��X��Ag+��ϳɲ�����ϡHNO3�İ�ɫ������A��B��E�е�XԪ�����ܡ�A�ڴ����ͼ��������¿ɷֽ�����C������F��F��ʹ�������ľ����ȼ��D��KOH��Һ��Ӧ����C��ˮ��B��A�����Ԫ����ȫ��ͬ����B��ˮ��Һ��ͨ��CO2����E������һ�����ʣ�E����ֽ�����D��F���Իش�
��1��д��A��B��C��D��E��F�������ʵĻ�ѧʽ��
A________��B________��C_______��D________��E________��F________��
��2��д������ָ����Ӧ�Ļ�ѧ����ʽ��
A�ֽ�����C��F��___________________________________��
E����ֽ⣺________________________________________��
���𰸡� KClO3 KClO KCl HCl HClO O2 2KClO3![]() 2KCl��3O2�� 2HClO
2KCl��3O2�� 2HClO![]() 2HCl��O2��
2HCl��O2��
����������1��F��ʹľ����ȼ����FΪ������������֪XΪ��Ԫ�أ�C��D�к���Cl����E����ֽ��D��F������EΪHClO��DΪHCl��D��KOH������C��ˮ������CΪKCl��A�ڼ��ȴ��������¿ɷֽ��C������F������AΪKClO3����B��ˮ��Һ��ͨ��CO2����E����һ��������B��A���Ԫ����ȫ��ͬ����BΪKCIO�����Ͽɵã�AΪKClO3��BΪKClO��CΪKCl��DΪHCl��EΪHClO��FΪO2��
��2��KClO3���ȷֽ�����KCl��O2����ѧ����ʽΪ��2KClO3![]() 2KCl��3O2����HClO����ֽ�����HCl��O2����ѧ����ʽΪ��2HClO
2KCl��3O2����HClO����ֽ�����HCl��O2����ѧ����ʽΪ��2HClO![]() 2HCl��O2����
2HCl��O2����