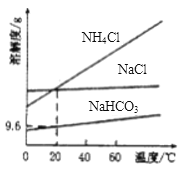
��Ŀ����
����Ŀ����HCl����������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.20g/cm3��������1L 1mol/L��ϡ���ᡣ�ش������й����⡣��������������裺
��1�����㣺����ȡ36.5%��Ũ��������Ϊ________������
��2����ȡ������Ͳ��ȡ����Ũ���Ტע�뵽250mL�ձ��У�
��3���ܽ⣺___________________________________________________����ȴ��
��4��ת�ƣ���Һ��:_________________________________________________��
��5��ϴ�ӣ�__________________________________________________________��
��6�����ݣ�__________________________________________________________��
��7��ҡ�ȣ��Ǻ�����ƿ���������ߵ���ҡ�ȣ�
��8�����أ������úõ�ϡ���ᵹ���Լ�ƿ�У������ñ�ǩ����ǩ��Ҫע��______________��
��9�������������²��������õ�����Ũ���ǡ�ƫ�ߡ�������ȡ����ǡ�ƫ�͡���
������Ͳ��ȡŨ�����������ˮϴ��Ͳ�������Һת������ƿ�У�____________��
������ƿ��������������ˮ��__________________��
��û�н�ϴ���ձ��Ͳ���������Һת������ƿ�У�___________��
�ܶ��ݶ���ʱ����������ƿ�Ŀ̶��ߣ�________________��
�ݶ���ҡ�Ⱥ�������ƿ��Һ����ڿ̶��ߣ��ּ�ˮ��________________��
��δ��ȴ��ת�ƶ��ݣ�_____________________��
����ϴ������ƿ��_______________��
���𰸡� 83.3 ��ʢ��Ũ������ձ��м���Լ100mLˮ���ò������������裬ʹ���Ͼ��� ���ձ��е���Һ�ز�����ת�Ƶ�����ƿ�� ����������ˮϴ���ձ��Ͳ�����2��3�Σ�����ϴ��ҺҲת�Ƶ�����ƿ��.Ȼ��ҡ�� ������ƿ�м�ˮ����Һ����̶�1--2cm����Һ��ӽ��̶��ߣ�ʱ���ý�ͷ�ι���εμ�ˮ����Һ����ʹ���̶������� ��ǩ��Ҫע��ϡ���ᡢ1mol/L ƫ�� ��� ƫ�� ƫ�� ƫ�� ƫ�� ƫ��
����������1����������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.20g/cm3�����ʵ���Ũ��C=![]() =12mol/L������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ�������ã�V��12mol/L=1mol/L��1000mL�����V=83.3mL��
=12mol/L������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ�������ã�V��12mol/L=1mol/L��1000mL�����V=83.3mL��
(3). ��ʢ��Ũ������ձ��м���Լ100mLˮ���ò������������裬ʹ���Ͼ��� (4). ���ձ��е���Һ�ز�����ת�Ƶ�����ƿ�� (5). ����������ˮϴ���ձ��Ͳ�����2��3�Σ�����ϴ��ҺҲת�Ƶ�����ƿ��.Ȼ��ҡ�� (6). ������ƿ�м�ˮ����Һ����̶�1--2cm����Һ��ӽ��̶��ߣ�ʱ���ý�ͷ�ι���εμ�ˮ����Һ����ʹ���̶������� (8). ��ǩ��Ҫע��ϡ���ᡢ1mol/L ��9����������������Ͳ��ȡŨ�����������ˮϴ��Ͳ�������Һת������ƿ�У�������ȡŨ�������ƫ������ƫ�࣬��ҺŨ��ƫ�ߣ�������ƿ��������������ˮ�������ʵ����ʵ�������Һ���������Ӱ�죬��ҺŨ����ȣ���û�н�ϴ���ձ��Ͳ���������Һת������ƿ�У����²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ܶ��ݶ���ʱ����������ƿ�Ŀ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ��ݶ���ҡ�Ⱥ�������ƿ��Һ����ڿ̶��ߣ��ּ�ˮ��������Һ���ƫ����ҺŨ��ƫ�ͣ���δ��ȴ��ת�ƶ��ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ�����ϴ������ƿ�������������ʵ���ƫ����ҺŨ��ƫ�ߣ�

 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�����Ŀ������ѧһѡ��3�����ʽṹ�����ʡ�
±��Ԫ�ص������������ʷ�Ӧ�γɶ��ֻ������������ѧ���ʽṹ�����ʵ����֪ʶ�ش�
(1)д����̬��ԭ�ӵļ۵����Ų�ʽ___________��
(2)±��Ԫ�صĺ�������������ǿ����____(д��ѧʽ)����������ӵ����幹��Ϊ_________��
(3)�Ƚ��������±������۵�ͷе㣬������仯���ɼ�ԭ��_________��
GeCl4 | GeBr4 | GeI4 | |
�۵�/�� | -49.5 | 26 | 146 |
�е�/�� | 83.1 | 186 | Լ400 |
(4)��֪�ߵ�����������ʽ����ѧʽ�ֱ�ΪH5IO6(![]() )��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��������ǿ��˳��ΪH5IO6_____ HIO4(�>������<����=��)��H5IO6�ЦҼ���м��ĸ�����Ϊ________��
)��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��������ǿ��˳��ΪH5IO6_____ HIO4(�>������<����=��)��H5IO6�ЦҼ���м��ĸ�����Ϊ________��
(5)��֪��Ԫ����������������ֻ��1�����ӡ�����������ԭ�ӹ�����������ӵ�Ԫ��M�γɵ�һ�ֻ����������������ͼ��ʾ��
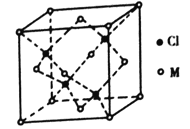
�ٸû�����Ļ�ѧʽΪ_______����֪��������a=0.542 nm���˾�����ܶ�Ϊ_____g/cm3��(д������ʽ����Ҫ��������������ӵ�����ΪNA)