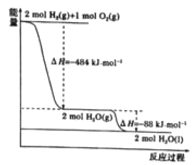
��Ŀ����
����Ŀ���ֱ���ʢ�Т���ɫʯ����Һ ��NaOH��Һ ��Ʒ����Һ �����Ը��������Һ���Թ���ͨ��SO2���塣
��1���Թܢ��е�����___________������Ӧ�Ļ�ѧ����ʽ�ǣ�_________________��
��2���Թܢ��з�����Ӧ�Ļ�ѧ����ʽ�ǣ�_____________________�����ͨ�������SO2��������Ӧ�Ļ�ѧ����ʽΪ��_______________________________��
��3���Թܢ��е�����________���罫����SO2��ĸ���Һ���ȣ�����______________��
��4���Թܢ��е�����____________________��
��5������ʵ���У�SO2���ֳ��������������ʵ���___________�����Թܱ�ţ���ͬ����SO2���ֳ�Ư���Ե���___________��SO2���ֳ���ԭ�Ե���___________��
���𰸡� ��� SO2+H2O ![]() H2SO3 SO2+2NaOH ��Na2SO3+H2O SO2+NaOH = NaHSO3 ��ɫ��ȥ �ָֻ���ɫ �Ϻ�ɫ��ʧ �٢� �� ��
H2SO3 SO2+2NaOH ��Na2SO3+H2O SO2+NaOH = NaHSO3 ��ɫ��ȥ �ָֻ���ɫ �Ϻ�ɫ��ʧ �٢� �� ��
����������1��SO2����������������ˮ��Ӧ����H2SO3����ɫʯ����Һ�����죬���Թܢ��е��������������Ӧ�Ļ�ѧ����ʽ����SO2+H2O ![]() H2SO3��
H2SO3��
��2��SO2�������������NaOH��Ӧ����Na2SO3��ˮ��SO2����������NaHSO3������Ӧ��ѧ����ʽΪ��SO2+2NaOH ��Na2SO3+H2O��SO2+NaOH = NaHSO3��
��3��SO2��Ư���Ա�����������ijЩ��ɫ���ʽ�ϳɲ��ȶ�����ɫ���ʣ���SO2ͨ��Ʒ����Һ����ɫ��ȥ�������ָֻ���ɫ��
��4��SO2���л�ԭ�ԣ���ǿ�����Ե����Ը��������Һ��Ӧ����ʹ���Ը��������Һ��ɫ��
��5���ۺ�����������SO2���ֳ��������������ʵ��Ǣ٢������ֳ�Ư���Ե��Ǣ������ֳ���ԭ�Ե��Ǣ���

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�����Ŀ����l����Cl2ͨ��ˮ�У�Cl2������ˮ��Ӧ��Cl2��H2O��H++Cl-+HClO����Ҫ����Cl2���ܽ⣬������ˮ�м�������________��
a.AgNO3���� b. CaCO3��ĩ c. NaCl���� d.����ˮ e��Ũ����
��2����һ�������£�ͬʱ����CO��H2O(g)��CO2��H2��һ�ܱ������з������·�Ӧ��CO +H2O(g)![]() CO2+H2����Ӧ��ʼʱ���ҽ��С�����˵���в���ȷ����__________��
CO2+H2����Ӧ��ʼʱ���ҽ��С�����˵���в���ȷ����__________��
a����Ӧ��ʼʱ������Ӧ���ʴ����淴Ӧ����
b����Ӧ��ʼʱ������Ӧ��������淴Ӧ����Ϊ��
c�����ŷ�Ӧ�Ľ��У�����Ӧ������С�����Ϊ��
d�����ŷ�Ӧ�Ľ��У��淴Ӧ������������Ӧ���ʼ�С�������ȡ�
��3��һ�������£��¶�ֵ����, SO2��O2��Ӧ��Ũ����ʱ��ı仯���±�
ʱ��(min) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
c(SO2)mol/L | 1.0 | |||||||
c(O2)mol/L | 0.5 | 0.35 | 0.25 | 0.18 | 0.1 | 0.05 | 0.05 | 0.05 |
C(SO3)mol/L | 0 | 0.3 | 0.5 | 0.65 | 0.8 | 0.9 | 0.9 | 0.9 |
���ϱ����ݼ��㣺
a.30min-40min ʱ��Σ���SO2��ʾ�ķ�Ӧ����Ϊ__________��
b.���¶��£�����Ӧ��ƽ�ⳣ����ֵΪ__________________��
c.ƽ��ʱ��SO2��ת����Ϊ_______��
��4���������[CO(NH3)2]�ļ�����Һ��������װ��ʾ��ͼ����ͼ�������и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫�������ʱ�������ĵ缫��ӦʽΪ____________��

��5��3.04gͭþ�Ͻ���ȫ�ܽ���100mL�ܶ�Ϊ1.40g/mL����������Ϊ63%�������У��õ�NO2����2688mL����״��������Ӧ�����Һ�м���1.0mol/L NaOH��Һ������������ȫ������ʱ���õ�5.08g�����������NaOH��Һ�������_________mL��
����Ŀ������ѧһѡ��3�����ʽṹ�����ʡ�
±��Ԫ�ص������������ʷ�Ӧ�γɶ��ֻ������������ѧ���ʽṹ�����ʵ����֪ʶ�ش�
(1)д����̬��ԭ�ӵļ۵����Ų�ʽ___________��
(2)±��Ԫ�صĺ�������������ǿ����____(д��ѧʽ)����������ӵ����幹��Ϊ_________��
(3)�Ƚ��������±������۵�ͷе㣬������仯���ɼ�ԭ��_________��
GeCl4 | GeBr4 | GeI4 | |
�۵�/�� | -49.5 | 26 | 146 |
�е�/�� | 83.1 | 186 | Լ400 |
(4)��֪�ߵ�����������ʽ����ѧʽ�ֱ�ΪH5IO6(![]() )��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��������ǿ��˳��ΪH5IO6_____ HIO4(�>������<����=��)��H5IO6�ЦҼ���м��ĸ�����Ϊ________��
)��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᣬ��������ǿ��˳��ΪH5IO6_____ HIO4(�>������<����=��)��H5IO6�ЦҼ���м��ĸ�����Ϊ________��
(5)��֪��Ԫ����������������ֻ��1�����ӡ�����������ԭ�ӹ�����������ӵ�Ԫ��M�γɵ�һ�ֻ����������������ͼ��ʾ��
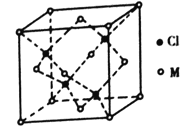
�ٸû�����Ļ�ѧʽΪ_______����֪��������a=0.542 nm���˾�����ܶ�Ϊ_____g/cm3��(д������ʽ����Ҫ��������������ӵ�����ΪNA)