��Ŀ����
����Ŀ��һ���ø�����(��Ҫ�ɷ�ΪMnCO3�Լ�Fe��Ba��Al������������P�����ؽ����ε�)�Ʊ�MnCl2�Ĺ����������£�

�ش��������⣺
(1)���ܽ���ʱ��Ϊ������̵Ľ�ȡ�ʣ��о�2����Ч�Ĵ�ʩ��___________��д���ò���MnCO3���뷴Ӧ�Ļ�ѧ����ʽ��__________��
(2)��������ʱ����Ҫ��Ӧ�����ӷ���ʽΪ______________��
(3)����2����Ҫ�ɷ�Ϊ______(�ѧʽ)������������������ԭ����______��
(4)��֪�����ؽ���������ܶȻ������
���� | MnS | PbS | CuS | NiS |
Ksp | 1.32��10-10 | 1.32��10-27 | 1.32��10-35 | 2.82��10-20 |
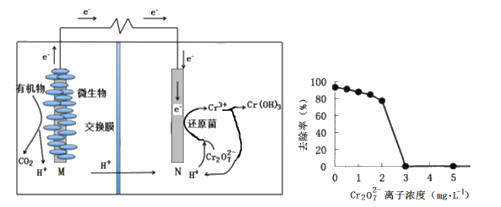
�������ؽ�����ʱ����Һ��pH����4.5��6.0��pH���˹��͵�ԭ����_______��
�����������ֽ�������Ũ����ͬ������Na2S��Һʱ���ȱ�������������_______����ӦMnS(s)��Cu2��(aq)![]() CuS(s)��Mn2��(aq)��ƽ�ⳣ��K��______��
CuS(s)��Mn2��(aq)��ƽ�ⳣ��K��______��
���𰸡��ʵ����������Ũ�Ⱥ��������ʵ���߽�ȡ�¶ȡ��ӳ���ȡʱ�䡢�����ĥ����ֱ����С�Ŀ����� MnCO3+2HCl=MnCl2+CO2��+2H2O MnO2+2Fe2++4H+=Mn2++2Fe3++ 2H2O Al(OH)3��Fe(OH)3 MnCO3������Һ�е�H+��ʹ��Һ�е�pH���ߣ��ٽ�Al3+��Fe3+ת��Ϊ���� ��ֹNa2S����H2S��ʧ Cu2+ 1��1025
��������
������(��Ҫ�ɷ�ΪMnCO3�Լ�Fe��Ba��Al������������P�����ؽ����ε�)������������������������ȫ���ܽ⣬����������̺������������ӣ�ͨ������pHʹ�����Ӻ������ӳ�����Ϊ����2���������������̳����������ᱵ�����ڱ����ӣ����������Ƴ�ȥ�ؽ������ӣ�������ȡ�ͷ���ȡ���ᾧ�����Ʊ�MnCl2��
(1)���ܽ���ʱ��Ϊ������̵Ľ�ȡ�ʣ������ʵ����������Ũ�Ⱥ��������ʵ���߽�ȡ�¶ȡ��ӳ���ȡʱ�䡢�����ĥ����ֱ����С�Ŀ����ȣ��ò���MnCO3�����ᷴӦ��ѧ����ʽ��MnCO3+2HCl=MnCl2+CO2��+2H2O��
(2)��������ʱ��������������Ϊ�����ӣ���Ҫ��Ӧ�����ӷ���ʽΪMnO2+2Fe2++4H+=Mn2++2Fe3++ 2H2O��
(3)������ҺpHֵ��������������������������������2����Ҫ�ɷ�ΪAl(OH)3��Fe(OH)3��������������ԭ����MnCO3������Һ�е�H+��ʹ��Һ�е�pH���ߣ��ٽ�Al3+��Fe3+ת��Ϊ������
(4�������ؽ�����ʱ����Һ��pH����4.5��6.0��������������������ӣ�pH���˹��͵�ԭ���Ƿ�ֹNa2S����H2S��ʧ��
�����������ֽ�������Ũ����ͬ�������ܶȻ���ϵ��֪���ܶȻ�ԽС��Խ���׳���������Na2S��Һʱ���ȱ�������������Cu2+����ӦMnS(s)��Cu2��(aq)![]() CuS(s)��Mn2��(aq)��ƽ�ⳣ��K��
CuS(s)��Mn2��(aq)��ƽ�ⳣ��K��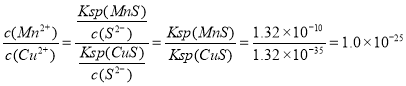 ��
��

 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�