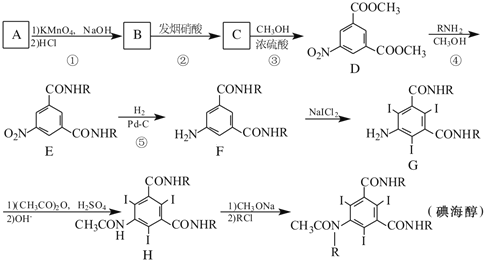
��Ŀ����
����Ŀ��ij����С���о���ͭ���Ϸ��ϣ������ڣ�����Ag2S�����������ʼ��Ʊ�����ͭ���壬�������£��������е��������������ϵͣ���ʵ��Ӱ��ɺ��ԣ�
��֪����AgCl�����ڰ�ˮ������[Ag(NH3)2]+��
��Ksp��Ag2SO4��=1.2��105��Ksp��AgCl��=1.8��1010��
�������к��������������������ܣ���
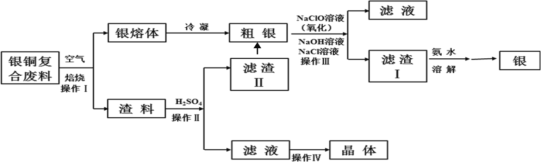
��1������I��Ϊ�˼ӿ�����������ʣ����Բ�ȡ_______��ʩ(д������һ�ּ���)��
��2������IV����________��_______���ˡ�ϴ�Ӻ�����Եõ�����ͭ���塣
��3��ϴ������II��������ϲ���Ŀ����__________��
��4������III�У�NaClO��Һ��Ag��Ӧ�IJ���ΪAgCl��NaOH��NaCl��O2���÷�Ӧ�Ļ�ѧ����ʽΪ______������AgCl��NaCl�����ʵ���֮��Ϊ2��1����������ͬʱ��������ת�������ӷ���ʽΪ_____����ƽ�ⳣ��K=__________��
��5���ڿ����м���5.20g����ͭ����(CuSO4��5H2O)��Ʒ�����ȹ����в�ͬ�¶ȷ�Χ�ڷֱ�õ�һ�ֹ������ʣ����������������֪CuSO4��5H2O��Է�������250��
�¶ȷ�Χ/�� | ��������/g |
200~260 | 3.33 |
650~800 | 1.67 |
1000~1500 | 1.50 |
���ȵ�1000��ʱ���������ʵĻ�ѧʽΪ��_______��
���𰸡�������ϡ��ʵ����ӿ������������ ����Ũ����������Ũ���� ���½ᾧ(����ȴ�ᾧ) Ϊ���Ag�Ļ����� 3NaClO+2Ag+H2O=2AgCl+NaCl+2NaOH+O2�� Ag2SO4(s)+2Cl(aq)![]() 2AgCl(s)+SO42(aq) 3.7��1014 Cu2O
2AgCl(s)+SO42(aq) 3.7��1014 Cu2O
��������
��1������������Ի�ѧ��Ӧ���ʵ�Ӱ�����������������ϡ��ʵ����ӿ�����������ȣ�
��2������IV�Ǵ���Һ�л������ͭ���壻
��3�������к������������ܵ���������������գ�
��4��NaClO��Һ������Ӧ�IJ���ΪAgCl��NaOH��O2����AgCl��NaCl�����ʵ���֮��Ϊ2��1����ϵ��ӵ�ʧ�غ��ԭ���غ���д��ѧ����ʽ��AgCl��Ag2SO4�ܽ�ȸ�С���ɴ�д������ת���Ļ�ѧ����ʽ������������ʵ�Ksp������ƽ�ⳣ��K��
��5������Cu�غ���㡣
��1������������Ի�ѧ��Ӧ���ʵ�Ӱ��������������I��Ϊ�˼ӿ�����������ʣ����Բ�ȡ������ϡ��ʵ����ӿ�����������ȣ�
��2����ͭ���Ϸ����ڿ����б��գ����е�Cuת����CuO����������Ҫ��CuO�����������������������м��������CuO�ܽ��CuSO4�������˺�õ�CuSO4��Һ������IV�Ǵ�CuSO4��Һ�л������ͭ���壬ͨ����������Ũ�������½ᾧ�����ˡ�ϴ�ӡ�����Ȳ��裬�Ϳ��Եõ�����ͭ���壻
��3�������к������������ܵ���������������գ�ϴ������II��������ϲ���Ŀ����Ϊ��������Ļ����ʣ�
��4��NaClO��Һ��Ag��Ӧ�IJ���ΪAgCl��NaOH��O2����AgCl��NaCl�����ʵ���֮��Ϊ2��1����ϵ�ʧ�����غ��ԭ���غ�ɵã�3NaClO+2Ag+H2O=2AgCl+NaCl+2NaOH+O2����AgCl��Ag2SO4�ܽ�ȸ�С���ɴ�д������ת�������ӷ���ʽ��Ag2SO4(s)+2Cl-(aq) ![]() 2AgCl(s)+SO42-(aq)���÷�Ӧ��ƽ�ⳣ��K=
2AgCl(s)+SO42-(aq)���÷�Ӧ��ƽ�ⳣ��K=![]() =
=![]() =
=![]() =3.7��1014��
=3.7��1014��
��5��5.20g����ͭ��������ʵ���Ϊ0.0208mol������CuSO4������Ϊ0.0208mol��160g/mol=3.33g��200~260�淶Χ�ڹ���ΪCuSO4��n��Cu��=0.0208mol��1000��ʱ��������Ϊ1.50g������n��O��=��1.50g-0.0208mol��64g/mol����16g/mol=0.01055mol��n��Cu����n��O��=2:1�����Լ��ȵ�1000��ʱʣ�����ΪCu2O��

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�����Ŀ����Դ����ϡ���Ϣ����Ϊ�ִ���ᷢչ������֧������ѧ����Դ����������ϵ��
(1)�±��е����ݱ�ʾ�ƻ�1mol��ѧ�������ĵ�����(�����ܣ���λΪkJmol��1)
��ѧ�� | H��H | Cl��Cl | H��Cl |
����/(kJmol��1) | 436 | 243 | 431 |
����������Ϣ��֪��1molH2��������������ȼ�������Ȼ�������ų���������_______
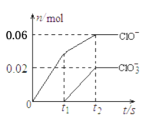
(2)��ҵ����һ�ַ�������CO2������ȼ�ϼ״������Խ�CO2���Ϊ���������Ϊ1L���ܱ������г���1molCO2��3molH2��һ�������·�����ӦCO2(g)+3H2(g)CH3OH(g)+H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
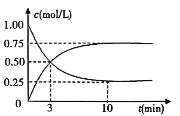
�ٴӷ�Ӧ��ʼ��ƽ�⣬CH3OH��ƽ����Ӧ������(CH3OH)=_______________��
������ӦCO2(g)+3H2(g)CH3OH(g)+H2O(g)�����ֲ�ͬ����µķ�Ӧ���ʷֱ�Ϊ��
A����(CO2)=0.15molL��1min��1 B����(H2)=0.01molL��1s��1
C����(CH3OH)=0.2molL��1min��1 D����(H2O)=0.45molL��1min��1
�÷�Ӧ�����ɿ쵽����˳��Ϊ___________(����ĸ)
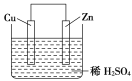
(3)��ˮ��ѧ��Դ�����þ��зdz�������ǰ�����Ӻ�ˮ����ȡ��Ĺ�ҵ������ͼ��ʾ��
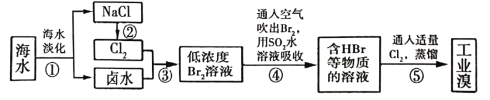
����������������漰������ԭ��Ӧ����_____________����
�ڲ�������ѻ������̬���壬���������֮ת��ɻ���̬���壬��Ŀ����__________��