��Ŀ����
����Ŀ��������FeCl3��Һ�����ˮʱ��Һ���Ϊ���ɫ���õ�����Fe(OH)3���塣�ô˷�ɢϵ����ʵ�飺
��1����д���Ʊ�Fe(OH)3����Ļ�ѧ����ʽ____________________________________________��
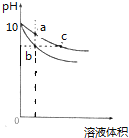
��2��֤���� Fe(OH)3 �������ɵ�ʵ���������һ���������Һ�壬���Կ����й�����ͨ·�����õĽ���������______________��
��3����Fe(OH)3 ����װ��U�ι��ڣ���ʯī���缫����ֱͨ����Դ��ͨ��һ��ʱ���������������ɫ������������_____��
��4����Fe(OH)3 ��������μ���ϡ���ᣬ���Կ����к��ɫ������������Ϊ���巢����_______�������μ�ϡ����ֱ�����������Կ�����ʵ��������________________________________________��
���𰸡�FeCl3+3H2O![]() Fe(OH)3(����) +3HCl�����ЧӦ��Ӿ�۳����ɫ�������ܽ�,��Һ��Ϊ��ɫ
Fe(OH)3(����) +3HCl�����ЧӦ��Ӿ�۳����ɫ�������ܽ�,��Һ��Ϊ��ɫ
��������
����Fe(OH)3(����) ������Ʊ�ʵ������𣻽����ж����ЧӦ���������磬�ܷ�����Ӿ������������ʿ��ܷ����۳���
��1��ʵ�����Ʊ����������������ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ������Һ��Ϊ���ɫʱ����ֹͣ���ȣ��Ʊ�Fe(OH)3����Ļ�ѧ����ʽ��FeCl3+3H2O![]() Fe(OH)3(����) +3HCl��
Fe(OH)3(����) +3HCl��
��2����һ���������ձ��еĽ��壬���Կ���������ͨ·�������ж����ЧӦ����Һû�У��ö����ЧӦ������������Һ�ͽ��壻
��3��Fe(OH)3 �����н��������磬��Fe(OH)3 ����װ��U�ι��ڣ���ʯī���缫����ֱͨ����Դ��ͨ��һ��ʱ���������������ɫ�����������е�Ӿ��
��4����Fe(OH)3 ��������μ���ϡ���ᣬ���Կ����к��ɫ������������Ϊ���巢���۳��������μ�ϡ����ֱ�����������Կ�����ʵ�������ǣ����ɫ�������ܽ�,��Һ��Ϊ��ɫ��

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�����Ŀ��������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50 mL 0.50 mol��L��1���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol��L��1 NaOH��Һ������ͬһ�¶ȼƲ�����¶ȣ��۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ���û��Һ����¶ȡ��ش��������⣺
��Ϊʲô����NaOH��ҺҪ�Թ�����__________________________________��
�ڵ���NaOH��Һ����ȷ������__________(����ĸ)��
A���ز������������� B���������������� C��һ��Ѹ�ٵ���
���ֽ�һ������ϡ����������Һ��ϡ����������Һ��ϡ��ˮ�ֱ��1 L 1 mol��L��1��ϡ����ǡ����ȫ��Ӧ���䷴Ӧ�ȷֱ�Ϊ��H1����H2����H3����H1����H2����H3�Ĵ�С��ϵΪ________________��
�ܼ������������������Һ���ܶȶ���1 g��cm��3����֪�кͷ�Ӧ��������Һ�ı�����c��4.18 J��g��1������1��Ϊ�˼����к��ȣ�ijѧ��ʵ���¼�������£�
ʵ����� | ��ʼ�¶�t1/ �� | ��ֹ�¶�t2/ �� | |
���� | ����������Һ | �����Һ | |
1 | 20.0 | 20.1 | 23.2 |
2 | 20.2 | 20.4 | 23.4 |
3 | 20.5 | 20.6 | 23.6 |
���ݸ�ѧ����ʵ�����ݼ��㣬��ʵ���õ��к��Ȧ�H��__________(�������һλС��)��
��________(��ܡ����ܡ�)��Ba(OH)2��Һ�������������������Һ�����ᣬ������______________________________________________________________________��