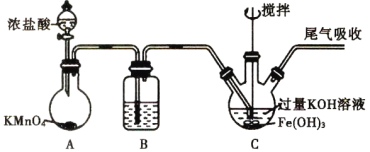
��Ŀ����
����Ŀ����������������Դ�����ˮ�����Ⱦ�ǻ�����������Ҫ�о����⡣
��1����֪����Ӧ�� N2(g)��O2(g)��2NO(g) ��H��a kJ��mol��1
��Ӧ�� 2NO(g)��O2(g)��2NO2(g) ��H��b kJ��mol��1
��Ӧ�� 4NH3(g)��5O2(g)��4NO(g)��6H2O(l) ��H��c kJ��mol��1
��д��NO2��NH3��Ӧ����N2��Һ̬ˮ���Ȼ�ѧ��Ӧ����ʽ______��
����Ӧ���и�����������ѧ�������ܺ��������±���a��______��
���� | N2 | O2 | NO |
ÿĦ���������������ܺ�/kJ��mol��1 | 946 | 498 | 630 |
��2���ô������Ƴ�ȥ������ԭ����ͼ��ʾ����ͼʾ���ܷ�Ӧ��ѧ����ʽΪ______��
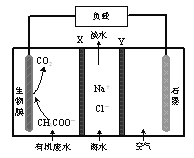
��3��ij����Ĥ���������õ��ԭ����������ˮ�е�NO![]() ��ԭΪN2������ԭ����ͼ��ʾ��
��ԭΪN2������ԭ����ͼ��ʾ��
��д�������ĵ缫��Ӧʽ��______��
�������ϣ�����ȥ0.04 mol NO![]() ������������������Ϊ______��(��״��)
������������������Ϊ______��(��״��)
���𰸡�6NO2(g)��8NH3(g)��7N2(g)��12H2O(l) ��H��(2c��7a��3b) kJ��mol��1 ��184 2NH3��3NaClO��N2��3NaCl��3H2O 2NO![]() ��10 e��6H2O��N2����12OH�� 1.12 L
��10 e��6H2O��N2����12OH�� 1.12 L
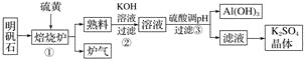
��������
��1���ٸ�����֪�Ȼ�ѧ����ʽ������ϸ�˹���ɽ��м��㣻
�ڸ�����H����Ӧ��ļ����ܺ�-������ļ����ܺͣ�
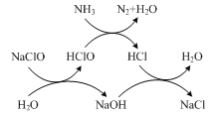
��2������ͼʾ����ʼ̬����̬�жϷ�Ӧ����NH3������ΪN2��NaClO����ԭΪNaCl������1molN2ת��6mol���ӣ����ݵ�ʧ�����غ�֪��Ҫ3molNaClO���ٽ��Ԫ���غ���ƽ�ܷ�Ӧ�Ļ�ѧ����ʽ��
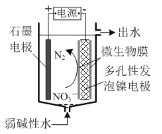
��3���ٸ�װ��Ϊ���أ����ص�����������ԭ��Ӧ����֪���ø�װ�ÿɽ�������ˮ�е�NO![]() ��ԭΪN2��NԪ�ػ��ϼ۽��ͣ�������ԭ��Ӧ����NO
��ԭΪN2��NԪ�ػ��ϼ۽��ͣ�������ԭ��Ӧ����NO![]() Ϊ������Ӧ�N2Ϊ�����
Ϊ������Ӧ�N2Ϊ�����
�ڸ�����������ת�Ƶ�������Ƚ��м��㡣
��1���ٸ�����֪�Ȼ�ѧ����ʽ����ϸ�˹���ɣ���֪ ��Ӧ���2-��Ӧ���3-��Ӧ���7�õ�NO2��NH3��Ӧ����N2��Һ̬ˮ���Ȼ�ѧ����ʽΪ��6NO2(g)��8NH3(g)��7N2(g)��12H2O(l) ��H��(2c��7a��3b) kJ��mol-1��
�ʴ�Ϊ��6NO2(g)��8NH3(g)��7N2(g)��12H2O(l) ��H��(2c��7a��3b) kJ��mol-1��
�ڸ�����H����Ӧ��ļ����ܺ�-������ļ����ܺͣ���֪��ӦI����H����946+498-2��630��kJ��mol��1=+184 kJ��mol��1����a=+184��
�ʴ�Ϊ��+184��
��2������ͼʾ����ʼ̬����̬�жϷ�Ӧ����NH3������ΪN2��NaClO����ԭΪNaCl������1molN2ת��6mol���ӣ����ݵ�ʧ�����غ�֪��Ҫ3molNaClO���ٽ��Ԫ���غ���ƽ�ܷ�Ӧ�Ļ�ѧ����ʽΪ��2NH3��3NaClO��N2��3NaCl��3H2O��
�ʴ�Ϊ��2NH3��3NaClO��N2��3NaCl��3H2O��
��3���ٸ�װ��Ϊ���أ����ص�����������ԭ��Ӧ����֪���ø�װ�ÿɽ�������ˮ�е�NO![]() ��ԭΪN2��NԪ�ػ��ϼ۽��ͣ�������ԭ��Ӧ����NO
��ԭΪN2��NԪ�ػ��ϼ۽��ͣ�������ԭ��Ӧ����NO![]() Ϊ������Ӧ�N2Ϊ������缫��ӦʽΪ��2NO
Ϊ������Ӧ�N2Ϊ������缫��ӦʽΪ��2NO![]() ��10 e��6H2O��N2����12OH����
��10 e��6H2O��N2����12OH����
�ʴ�Ϊ��2NO![]() ��10 e��6H2O��N2����12OH����
��10 e��6H2O��N2����12OH����
����֪������Ӧʽ2NO![]() ��10 e��6H2O��N2����12OH�����������ϣ�����ȥ0.04 mol NO
��10 e��6H2O��N2����12OH�����������ϣ�����ȥ0.04 mol NO![]() ��ת��0.2mol���ӣ���������������������1mol����ת��4mol���ӣ���ת��0.2mol���ӣ���������0.05mol��V=0.05mol��22.4L/mol=1.12 L��
��ת��0.2mol���ӣ���������������������1mol����ת��4mol���ӣ���ת��0.2mol���ӣ���������0.05mol��V=0.05mol��22.4L/mol=1.12 L��
�ʴ�Ϊ��1.12 L��

 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�