��Ŀ����
��֪���ٽ�úת��Ϊˮú������Ҫ��ѧ��ӦΪC(s)��H2O(g) CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
C(s)��O2(g)=CO2 (g)����H����393.5 kJ��mol��1
CO(g)��1/2O2(g)=CO2(g)����H����283.0 kJ��mol��1
H2(g)��1/2O2(g)=H2O(g)����H����242.0 kJ��mol��1
��ش�
(1)����������Ϣ��д��CO��ˮ������Ӧ���Ȼ�ѧ����ʽ��____________________________��

(2)��ͼ�Ǹ��ݸ�˹����������ѭ��ͼ������ͼ��ת����ϵ���Ȼ�ѧ����ʽ���㦤H3��________kJ/mol��
��ȽϦ�H1�릤H3��ֵ�Ƿ����˵����ˮú����ȼ��Ҫ��ֱ��ȼú�ų���������________(�ǻ��)ԭ����___________________________________��
(3)Ŀǰú�����仹��Ҫ����·�����·���䣬��������ѧ֪ʶ���������������·��·����ķ�����____________________________��
 CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��C(s)��O2(g)=CO2 (g)����H����393.5 kJ��mol��1
CO(g)��1/2O2(g)=CO2(g)����H����283.0 kJ��mol��1
H2(g)��1/2O2(g)=H2O(g)����H����242.0 kJ��mol��1
��ش�
(1)����������Ϣ��д��CO��ˮ������Ӧ���Ȼ�ѧ����ʽ��____________________________��

(2)��ͼ�Ǹ��ݸ�˹����������ѭ��ͼ������ͼ��ת����ϵ���Ȼ�ѧ����ʽ���㦤H3��________kJ/mol��
��ȽϦ�H1�릤H3��ֵ�Ƿ����˵����ˮú����ȼ��Ҫ��ֱ��ȼú�ų���������________(�ǻ��)ԭ����___________________________________��
(3)Ŀǰú�����仹��Ҫ����·�����·���䣬��������ѧ֪ʶ���������������·��·����ķ�����____________________________��
(1)CO(g)��H2O(g)=CO2(g)��H2(g)����H����41.0 kJ/mol
(2)���ݸ�˹����û�м��㦤H2���յ�����
(3)��ú��Ϊˮú����ܵ�����
(2)���ݸ�˹����û�м��㦤H2���յ�����
(3)��ú��Ϊˮú����ܵ�����
(1)CO(g)��H2O(g)=CO2(g)��H2(g)����H����41.0 kJ/mol
(2)��H3����525.0 kJ/mol����H1����393.5 kJ/mol��������Ϊ��H3��ֵ���ڦ�H1��˵��ˮú����ȼ�Ϸų������࣬��Ϊ��ԭ����H2������û�м��㡣
(3)��ú��Ϊˮú�����ùܵ����䣬�ɻ�����·��·����ѹ����
(2)��H3����525.0 kJ/mol����H1����393.5 kJ/mol��������Ϊ��H3��ֵ���ڦ�H1��˵��ˮú����ȼ�Ϸų������࣬��Ϊ��ԭ����H2������û�м��㡣
(3)��ú��Ϊˮú�����ùܵ����䣬�ɻ�����·��·����ѹ����

��ϰ��ϵ�д�
�����Ŀ
 2CO2(g) +4H2O(g) ?H1= ��1275��6 kJ/mol
2CO2(g) +4H2O(g) ?H1= ��1275��6 kJ/mol  CH3OH(g)+ H2O(g) ?H = ��49 kJ/mol
CH3OH(g)+ H2O(g) ?H = ��49 kJ/mol 
 2H2(g)+O2(g)����H1
2H2(g)+O2(g)����H1 =
= mol
mol
 =
=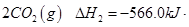 mol
mol =
= mol
mol
 ��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����
��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼa����
 (�<������>����="����ͬ)��A��B��C���㴦��Ӧƽ�ⳣ��(
(�<������>����="����ͬ)��A��B��C���㴦��Ӧƽ�ⳣ��( )�Ĵ�С��ϵΪ ��
)�Ĵ�С��ϵΪ ��
 ͨ���ݻ�Ϊ1L�Ķ����ܱ������з�����Ӧ����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� (�����)��
ͨ���ݻ�Ϊ1L�Ķ����ܱ������з�����Ӧ����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬���� (�����)��
 ��KOH(aq)��Ƴ���ͼb��ʾ�ĵ��װ�ã���õ�ظ����ĵ缫��ӦʽΪ ��
��KOH(aq)��Ƴ���ͼb��ʾ�ĵ��װ�ã���õ�ظ����ĵ缫��ӦʽΪ ��
 O2��HbCO��ʵ�������c(HbCO)��ʹֻ��c(HbO2)��
O2��HbCO��ʵ�������c(HbCO)��ʹֻ��c(HbO2)�� ��Ҳ������˵��������ˡ���֪t ��ʱ������Ӧ��ƽ�ⳣ��K��200������β�O2��Ũ��ԼΪ1.0��10��2 mol��L��1����ʹc(HbCO)С��c(HbO2)��
��Ҳ������˵��������ˡ���֪t ��ʱ������Ӧ��ƽ�ⳣ��K��200������β�O2��Ũ��ԼΪ1.0��10��2 mol��L��1����ʹc(HbCO)С��c(HbO2)�� CO2 (g)+ H2(g) ��H 2 = ?41 kJ/mol����ʼʱ���ܱ������г���1.00 molCO��1.00 molH2O���ֱ��������ʵ�飬̽��Ӱ��ƽ������أ�����������ͬ�Ҳ������κθ���Ӧ��Ӱ�죩��ʵ���������±���
CO2 (g)+ H2(g) ��H 2 = ?41 kJ/mol����ʼʱ���ܱ������г���1.00 molCO��1.00 molH2O���ֱ��������ʵ�飬̽��Ӱ��ƽ������أ�����������ͬ�Ҳ������κθ���Ӧ��Ӱ�죩��ʵ���������±���
 (m��x)CeO2��xCe��xO2
(m��x)CeO2��xCe��xO2 mCeO2��xH2��xCO
mCeO2��xH2��xCO
 O2(g)=FeO(s)����H����272.0 kJ��mol��1
O2(g)=FeO(s)����H����272.0 kJ��mol��1 O2(g)=Al2O3(s)����H����1675.7 kJ��mol��1
O2(g)=Al2O3(s)����H����1675.7 kJ��mol��1
 Na2S(s)��4H2O(g)
Na2S(s)��4H2O(g)