��Ŀ����
ú�Ʊ�CH4��һ���з�չǰ�����¼�����
I. ú̿�������Ʊ�CH4�������·�Ӧ:
C(s)+H2O(g)=CO(g)+H2 (g) ��H 1 = +131 kJ/mol
CO(g) + H2O(g)=CO2 (g)+ H2(g) ��H 2 = ?41 kJ/mol
CO(g) + 3H2 (g)=CH4 (g)+ H2O(g) ��H 3 = ?206 kJ/mol
(1)д��ú����̬ˮ�Ʊ�CH4(���ﻹ��CO2)���Ȼ�ѧ����ʽ ��
(2)úת��Ϊˮú��(CO��H2)��Ϊȼ�Ϻ�úֱ����Ϊȼ����ȣ���Ҫ���ŵ��� ��
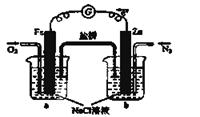
(3)д�����顪����ȼ�ϵ��(�������ҺΪKOH��Һ)�и����ĵ缫��Ӧʽ ��
II. �����Ϸ�ӦCO(g) + H2O(g) CO2 (g)+ H2(g) ��H 2 = ?41 kJ/mol����ʼʱ���ܱ������г���1.00 molCO��1.00 molH2O���ֱ��������ʵ�飬̽��Ӱ��ƽ������أ�����������ͬ�Ҳ������κθ���Ӧ��Ӱ�죩��ʵ���������±���
CO2 (g)+ H2(g) ��H 2 = ?41 kJ/mol����ʼʱ���ܱ������г���1.00 molCO��1.00 molH2O���ֱ��������ʵ�飬̽��Ӱ��ƽ������أ�����������ͬ�Ҳ������κθ���Ӧ��Ӱ�죩��ʵ���������±���
(1)ʵ�����c(CO2)��ʱ��仯�Ĺ�ϵ����ͼ�����ڴ���Ŀ�ͼ�У�����ʵ��ں͢���c(CO2)��ʱ��仯��ϵ��Ԥ�ڽ��ʾ��ͼ��

(2)����ʵ�����ͬ�������£���ʼʱ�������������ʵ�����n(CO)=n(H2O)=n(CO2) =n( H2)=1.00mol��ͨ�����㣬�жϳ���Ӧ���еķ���д��������̡���
I. ú̿�������Ʊ�CH4�������·�Ӧ:
C(s)+H2O(g)=CO(g)+H2 (g) ��H 1 = +131 kJ/mol
CO(g) + H2O(g)=CO2 (g)+ H2(g) ��H 2 = ?41 kJ/mol
CO(g) + 3H2 (g)=CH4 (g)+ H2O(g) ��H 3 = ?206 kJ/mol
(1)д��ú����̬ˮ�Ʊ�CH4(���ﻹ��CO2)���Ȼ�ѧ����ʽ ��
(2)úת��Ϊˮú��(CO��H2)��Ϊȼ�Ϻ�úֱ����Ϊȼ����ȣ���Ҫ���ŵ��� ��
(3)д�����顪����ȼ�ϵ��(�������ҺΪKOH��Һ)�и����ĵ缫��Ӧʽ ��
II. �����Ϸ�ӦCO(g) + H2O(g)
 CO2 (g)+ H2(g) ��H 2 = ?41 kJ/mol����ʼʱ���ܱ������г���1.00 molCO��1.00 molH2O���ֱ��������ʵ�飬̽��Ӱ��ƽ������أ�����������ͬ�Ҳ������κθ���Ӧ��Ӱ�죩��ʵ���������±���
CO2 (g)+ H2(g) ��H 2 = ?41 kJ/mol����ʼʱ���ܱ������г���1.00 molCO��1.00 molH2O���ֱ��������ʵ�飬̽��Ӱ��ƽ������أ�����������ͬ�Ҳ������κθ���Ӧ��Ӱ�죩��ʵ���������±���| ʵ���� | �������/L | �¶�/��C |
| �� | 2.0 | 1200 |
| �� | 2.0 | 1300 |
| �� | 1.0 | 1200 |
(1)ʵ�����c(CO2)��ʱ��仯�Ĺ�ϵ����ͼ�����ڴ���Ŀ�ͼ�У�����ʵ��ں͢���c(CO2)��ʱ��仯��ϵ��Ԥ�ڽ��ʾ��ͼ��

(2)����ʵ�����ͬ�������£���ʼʱ�������������ʵ�����n(CO)=n(H2O)=n(CO2) =n( H2)=1.00mol��ͨ�����㣬�жϳ���Ӧ���еķ���д��������̡���
I.��1��2C(s)+ 2H2O(g)= CH4 (g)+ CO2 (g) ��H = +15 kJ/mol ��3�� ��
(2)���ú�������ʣ���Լ��Դ���Լ��Դ����1�֣���������Ⱦ����1�֣�
(3)CH4��8e-+10OH-=CO32-+7H2O��2�֣�
II. (1) ��ͼ��ʾ

(2)Q�� K ��Ӧ���淴Ӧ��������CO��H2O�ķ����� ��1�֣�
(2)���ú�������ʣ���Լ��Դ���Լ��Դ����1�֣���������Ⱦ����1�֣�
(3)CH4��8e-+10OH-=CO32-+7H2O��2�֣�
II. (1) ��ͼ��ʾ

(2)Q�� K ��Ӧ���淴Ӧ��������CO��H2O�ķ����� ��1�֣�
��������� I. (1) ���ݸ�˹���ɺ���֪����ʽ��ú����̬ˮ�Ʊ�������Ȼ�ѧ����ʽ���ɷ���ʽ�١�2+��+�۶��ã����Ԧ�H=2��H 1+ ��H 2+��H 3 = +15 kJ/mol �������Ȼ�ѧ����ʽΪ��2C(s)+ 2H2O(g)= CH4 (g)+ CO2 (g) ��H = +15 kJ/mol ��
(2) ú����������ˮú����ȼ�ձ�ֱ��ȼ��ú���ӳ�֣������Դ�����ʣ�ͬʱȼú���ײ������꣬����ȼ��ˮú�������Լ�����Ⱦ��
(3)�ü�����ȼ���Ƴɵļ���ȼ�ϵ���У�����ʧ�������ɵ�CO2���������Һ�еļ��Խ��ʽ�һ����Ӧ����CO32-�������为���ķ�ӦʽΪ��CH4��8e-+10OH-=CO32-+7H2O��
II. (1) CO(g) + H2O(g)
 CO2 (g)+ H2(g) �ķ�Ӧ��һ�����ȷ�Ӧ�������¶����ߣ���Ӧ�ķ�Ӧ���ʼӿ죬��ʹƽ�������ƶ���ʵ������߱�ʵ������߸���ﵽƽ�⣬����ƽ��ʱCO2��Ũ��Ҫ��һЩ�����֮�£�ʵ��۱��������С���������������ѹǿ�����ڷ�Ӧǰ������ϵ�����䣬��������ѹǿֻ������ѧ��Ӧ���ʣ����Dz��ܸı�ƽ�⣬���ʵ��۵����߱�ʵ��ٸ���ﵽƽ�⣬��ƽ��ʱCO2��Ũ����ʵ��ٵ�2������ͼ
CO2 (g)+ H2(g) �ķ�Ӧ��һ�����ȷ�Ӧ�������¶����ߣ���Ӧ�ķ�Ӧ���ʼӿ죬��ʹƽ�������ƶ���ʵ������߱�ʵ������߸���ﵽƽ�⣬����ƽ��ʱCO2��Ũ��Ҫ��һЩ�����֮�£�ʵ��۱��������С���������������ѹǿ�����ڷ�Ӧǰ������ϵ�����䣬��������ѹǿֻ������ѧ��Ӧ���ʣ����Dz��ܸı�ƽ�⣬���ʵ��۵����߱�ʵ��ٸ���ﵽƽ�⣬��ƽ��ʱCO2��Ũ����ʵ��ٵ�2������ͼ
(2)����˼·���ȸ���ʵ��ٳ�������ʵ����ʵ�����ƽ��ʱ��CO2��Ũ�ȼ��������ͬ�¶��µ�ƽ�ⳣ��K��Ȼ��Ƚϴ�ʱ��Ũ����Q��ʵ��ٵ�Kֵ�Ĵ�С���Ӷ��ó���Ӧ���еķ���
���������
(2) ƽ��ʱn(CO2)=0.20mol/Lx2.0L=0.40mol
CO(g) + H2O(g)
 CO2 (g)+ H2(g)
CO2 (g)+ H2(g) ��ʼ���ʵ���/mol 1.00 1.00 1.00 1.00
�仯���ʵ���/mol 0.40 0.40 0.40 0.40
ƽ�����ʵ���/mol 0.60 0.60 0.40 0.40 ��1�֣�
K=
 ="0.44" ��2�֣�
="0.44" ��2�֣�Q=
 =
= =" 1.0" ��1�֣�
=" 1.0" ��1�֣�Q�� K ��Ӧ���淴Ӧ��������CO��H2O�ķ����� ��1�֣�

��ϰ��ϵ�д�
�����Ŀ
 C6 H5- CH=CH2 (g) +H2(g) ��H1
C6 H5- CH=CH2 (g) +H2(g) ��H1
 CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
 CH3OH(g)����H1
CH3OH(g)����H1
 Fe3+(aq)+3OH-(aq) �� ��H=" a" kJ?mol-1
Fe3+(aq)+3OH-(aq) �� ��H=" a" kJ?mol-1 CH3OCH3��H2O
CH3OCH3��H2O CH3OCH3(g)��CO2(g) ��H����247kJ/mol
CH3OCH3(g)��CO2(g) ��H����247kJ/mol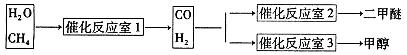
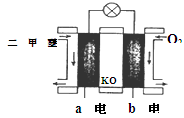

 2H2��+O2��
2H2��+O2�� 2H2��+O2��
2H2��+O2�� 2H2��+O2��
2H2��+O2�� CO+3H2
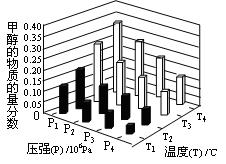
CO+3H2 CH3OH��g��+H2O��g������H="-49.0" kJ/mol,���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
CH3OH��g��+H2O��g������H="-49.0" kJ/mol,���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
 CH3OH��g����ƽ�ⳣ��:
CH3OH��g����ƽ�ⳣ��:

