��Ŀ����
18��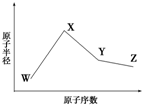 ��֪W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Y��Zͬ���ڣ�����X��M���������K���һ�룻Y�ĵ�����һ�ֳ����İ뵼��
��֪W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯��ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Y��Zͬ���ڣ�����X��M���������K���һ�룻Y�ĵ�����һ�ֳ����İ뵼�����ϣ�Z�ĵ縺����ͬ��������Ԫ�������
��1��XԪ����Ԫ�����ڱ���λ�õ������ڵ�IA�壻Z�Ļ�̬ԭ�Ӻ�����1��δ�ɶԵ��ӣ�
��2��X�ĵ��ʺ�Y�ĵ�����ȣ��۵�ϸߵ���Si��д��ѧʽ����Z����̬�⻯����廯����ȣ����ȶ�����HCl��д��ѧʽ����
��3��Y��Z�γɵĻ����������ˮ��Ӧ������һ�������һ��ǿ�ᣬ�÷�Ӧ�Ļ�ѧ����ʽ��SiCl4+3H2O=H2SiO3��+4HCl��
��4����25�桢101kPa�£���֪Y����̬�⻯������������ȫȼ�պ�ָ���ԭ״̬��ƽ��ÿת��1mol���ӷ���190.0kJ���÷�Ӧ���Ȼ�ѧ����ʽ��SiH4��g��+2O2��g���TSiO2��s��+2H2O��l����H=-1520.0kJ•mol-1��
���� ��W��һ�ֺ��ص�������Ϊ18��������Ϊ10����֪W��������Ϊ8��ΪOԪ�أ�X��Y��Zͬ���ڣ�����X��M���������K���һ�룬��X����3�����Ӳ㣬����㺬��1�����ӣ���XΪNaԪ�أ�Y�İ뾶����X��W֮�䣬Y�ĵ�����һ�ֳ����İ뵼����ϣ���Y��SiԪ�أ�Z��ԭ�Ӱ뾶��ͬ��������Ԫ������С��Z��X����ͬһ���ڣ���Ϊ��������Ԫ�أ�����ZΪ��Ԫ�أ��ݴ˽��н��
��� �⣺��W��һ�ֺ��ص�������Ϊ18��������Ϊ10����֪W��������Ϊ8��ΪOԪ�أ�X��Y��Zͬ���ڣ�����X��M���������K���һ�룬��X����3�����Ӳ㣬����㺬��1�����ӣ���XΪNaԪ�أ�Y�İ뾶����X��W֮�䣬Y�ĵ�����һ�ֳ����İ뵼����ϣ���Y��SiԪ�أ�Z��ԭ�Ӱ뾶��ͬ��������Ԫ������С��Z��X����ͬһ���ڣ���Ϊ��������Ԫ�أ�����ZΪ��Ԫ�أ�
��1��XΪ��Ԫ�أ�ԭ������Ϊ11��λ�����ڱ��е������ڵ�IA�壻��������Ϊ8��ZΪClԪ�أ���̬ԭ���Ų�ʽΪ1s22s22p63s23p5�����⺬��1��δ�ɶԵ��ӣ�
�ʴ�Ϊ���������ڵ�IA�壻1��
��2��X��Na��Y��Si���ǽ�����Cl��Br�����⻯���ȶ���HCl��HBr��
�ʴ�Ϊ��Si��HCl��
��3��Y��Z�γɵĻ�����ΪSiCl4��������ˮ��Ӧ������һ�������һ��ǿ�ᣬӦ���ɹ�����HCl���÷�Ӧ�Ļ�ѧ����ʽ��SiCl4+3H2O=H2SiO3��+4HCl��
�ʴ�Ϊ��SiCl4+3H2O=H2SiO3��+4HCl��
��4��Y�ǹ�Ԫ�أ���ΪSiH4+2O2�TSiO2+2H2O��8e-�����ԣ�����1molSiH4�μӷ�Ӧ�ǣ��ų�����Ϊ190.0kJ��8=1520.0 kJ�����ȷ�Ӧ����ʽΪ��SiH4��g��+2O2��g���TSiO2��s��+2H2O��l����H=-1520.0 kJ•mol-1��
�ʴ𰸣�SiH4��g��+2O2��g���TSiO2��s��+2H2O��l����H=-1520.0 kJ•mol-1��
���� ���⿼����λ�á��ṹ�����ʹ�ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ���Ҫ����ѧ������Ԫ�ص�ԭ�ӽṹ�ص���Ԫ�����࣬�ٸ����������ڱ��е�λ�ã������ʣ������õ���ת����Ŀ���з�Ӧ�ȵļ��㣬������ѧ�������ļ����������淶��д�ȷ���ʽԭ��

 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
��1��ͼ1�Dz��ֶ�����Ԫ��ԭ�ӣ�����ĸ��ʾ��������������ԭ�������Ĺ�ϵͼ��
��YԪ����Ԫ�����ڱ��е�λ��Ϊ�ڶ����ڵڢ���A�壮
����̬�⻯����ȶ��ԣ�X�����������������Y��
��������Ԫ���γɵļ������У����Ӱ뾶������S2-�������ӷ��ţ���
��Z���⻯��ZH���Ժ�ˮ����������ԭ��Ӧ���䷴Ӧ����ʽΪNaH+H2O=NaOH+H2����
��2��ͼ2�Ǽס��ҡ��������������ʵ�ת����ϵ������ÿһ������һ��ʵ�ֵ���B
| �� | �� | �� | �� | |
| A | FeCl3 | FeCl2 | Fe2O3 | Fe��OH��3 |
| B | Cu | CuO | CuSO4 | CuCl2 |
| C | NO | HNO3 | NO2 | NH3 |
| D | Si | Na2SiO3 | SiO2 | SiF4 |
| A�� | R�������ﶼ������ˮ | |
| B�� | R������������Ӧ��ˮ���ﶼ��H2RO3 | |
| C�� | R�Ƿǽ���Ԫ�� | |
| D�� | R�������ﶼ����NaOH��Һ��Ӧ |
| A�� | ������ӦʽΪ��O2+2H2O+4e-�T4OH- | |
| B�� | ����һ��ʱ����Һ��KOH�����ʵ������� | |
| C�� | ��ȼ�ϵ�ص��ܷ�Ӧ����ʽΪ��2H2+O2�T2H2O | |
| D�� | H2ͨ���һ��Ϊ���� |
| A�� | Ԫ��W �ļ���̬�⻯������ȶ��Ա�X ��ǿ | |
| B�� | Ԫ��W ������������Ӧˮ��������Ա�Z ���� | |
| C�� | ������YX��ZX2��WX3 �л�ѧ����������ͬ | |
| D�� | ԭ�Ӱ뾶�Ĵ�С˳��rY��rZ��rW��rX |
| A�� | ${\;}_{53}^{127}$I��${\;}_{53}^{131}$I��${\;}_{55}^{134}$Cs��${\;}_{55}^{137}$Cs�����ֺ��أ�����ͬλ�� | |
| B�� | ${\;}_{53}^{131}$I��${\;}_{55}^{137}$Cs���������ֱ���78��82 | |
| C�� | �����ڱ���Cs��Iλ��ͬһ���� | |
| D�� | �����ܻ�������CsI |
| A�� | ��CH3��2CHCH2CH��CH3��CH��CH3��2 2��4��5-�������� | |
| B�� | 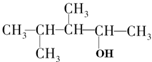 1��2��3-����-1-���� 1��2��3-����-1-���� | |
| C�� | CH2=C��CH3��CH2C��CH3��32��4��4-����-1-��ϩ | |
| D�� | CH3COOCH2CH2OOCCH3 �Ҷ����Ҷ��� |
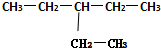 ϵͳ��������3-�һ�����
ϵͳ��������3-�һ�����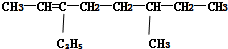 ϵͳ��������6-��-3-�һ�-2-��ϩ
ϵͳ��������6-��-3-�һ�-2-��ϩ