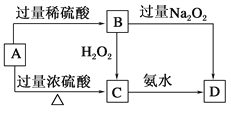
��Ŀ����
����Ŀ��������Ⱦ�����Чȥ������Դ�ij�������ǻ�ѧ�츣�������Ҫ�о����⡣
��1������β���е�CO��NOx��̼�⻯�����Ǵ�����Ⱦ�
��ʹ��ϡ���ȴ����ܽ�CO��NOת���������ʡ�
��֪��N2(g)��O2(g)=2NO(g) ��H1����180.5 kJ��mol��1
2C(s)��O2(g)=2CO(g)�� ��H2����221 kJ��mol��1
C(s)��O2(g)=CO2(g)�� ��H3����393.5 kJ��mol��1
д��NO(g)��CO(g)��ת����N2(g)��CO2(g)���Ȼ�ѧ����ʽ��_____________________��
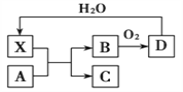
��ȩ�ࡢO3��PAN(������������)����Ⱦ������Ϳ��������γɵ�������Ϊ�⻯ѧ������ij�о���ѧϰС��Ϊģ��⻯ѧ�������γɣ�������������װ���ܱ������ڵı���Ⱦ������Ʒ���������ʵ�Ũ����ʱ��ı仯��ͼ1��ʾ��������ݹ⻯ѧ�������γ�ԭ�����Լ��ٹ⻯ѧ�����ķ������һ���������飺________________________________��
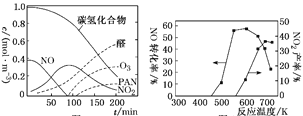
ͼ1 ͼ2
��2������NH3����ԭ��������(SCR����)��ĿǰӦ�ù㷺����������������SCR��������Ҫ��Ӧ֮һΪ4NO(g)��4NH3(g)��O2(g) ![]() 4N2(g)��6H2O(g)��H����1627.2 kJ��mol��1��NO��NH3������������Ag2O�������£�����Ӧ�¶����ߵ�550��700 ����NOת���������½���NO2������������(��ͼ2)�Ŀ���ԭ����__________________________��(�û�ѧ����ʽ����)
4N2(g)��6H2O(g)��H����1627.2 kJ��mol��1��NO��NH3������������Ag2O�������£�����Ӧ�¶����ߵ�550��700 ����NOת���������½���NO2������������(��ͼ2)�Ŀ���ԭ����__________________________��(�û�ѧ����ʽ����)
��3��Ŀǰ����ѧ�һ����о�һ������ϩ��Ϊ��ԭ��������(NO)ԭ��������������ʾ��ͼ��ͼ3�����������¶ȡ�������(����ɸ�д�������������)�Ĺ�ϵ��ͼ4��ʾ��
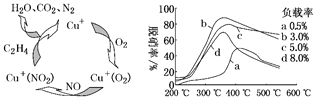
ͼ3 ͼ4
��д��������ԭ���ܷ�Ӧ�Ļ�ѧ����ʽ_____������֪NO��C2H4�����Ϊ3:1��
��Ϊ�ﵽ�������Ч����Ӧѡ���������_________________________________��
��4��NO(g)��CO(g)��ת����N2(g)��CO2(g)�ķ�Ӧ�ﵽƽ���20minʱ�����ı䷴Ӧ����������N2Ũ�ȷ�������ͼ5��ʾ�ı仯����ı������������________(����ĸ)��
A����������� B�������¶ȡ� C������CO2���� D����С�������
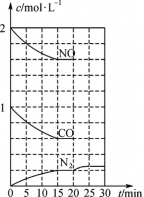
ͼ5 ͼ6
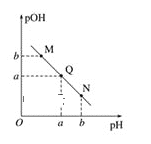
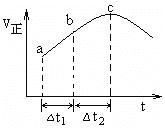
��5����һ�������£���SO2��NO2ͨ����Ⱥ����ܱ������У�������Ӧ��SO2(g)+NO2(g) ![]() SO3(g) +NO(g)������Ӧ������ʱ��仯��ͼ6��ʾ����ͼ�ɵó�����ȷ������___(����ĸ)
SO3(g) +NO(g)������Ӧ������ʱ��仯��ͼ6��ʾ����ͼ�ɵó�����ȷ������___(����ĸ)
A����Ӧ��c��ﵽƽ��״̬
B����Ӧ��Ũ�ȣ�b��С��c��
C����Ӧ��������������������������
D����t1=��t2ʱ��SO2��ת���ʣ�a��b��С��b��c��
���𰸡�2NO(g)��2CO(g)===N2(g)��2CO2(g)��H����746.5 kJ��mol��1 ���ٻ������к�β�����ŷ� 4NH3��7O2 4NO2��6H2O 6NO��3O2��2C2H4
4NO2��6H2O 6NO��3O2��2C2H4 3N2��4CO2��4H2O ԼΪ350 �桢������3.0% B D
3N2��4CO2��4H2O ԼΪ350 �桢������3.0% B D
��������
��1����N2(g)��O2(g)=2NO(g) ��H1����180.5 kJ��mol��1��
2C(s)��O2(g)=2CO(g)�� ��H2����221 kJ��mol��1��
C(s)��O2(g)=CO2(g)�� ��H3����393.5 kJ��mol��1��
����ʽ����2-��-�ٵ�2NO(g)+2CO(g)=N2(g)+2CO2(g)
������H=��-393.5kJ/mol����2-��-221.0kJ/mol��-��+180.5kJ/mol��=-746.5 kJ/mol��
�ʴ�Ϊ��2NO(g)+2CO(g)=N2(g)+2CO2(g) ��H=-746.5 kJ/mol��
����ͼ��֪������ʱ��ļӳ������ࡢ������������ת��Ϊȩ��PAN�����������γɹ⻯ѧ���������ٹ⻯ѧ�����ķ������ɼ��ٵ�����������ŷţ�����ٻ������к�β�����ŷţ�
��2����Ӧ4NO(g)��4NH3(g)��O2(g) ![]() 4N2(g)��6H2O(g)��H����1627.2 kJ��mol��1�Ƿ��ȷ�Ӧ������ƽ�������ƶ����¶�����ʱ���ᷢ�������Ĵ�������Ӧ����NO2����Ӧ����ʽ��4NH3��7O2
4N2(g)��6H2O(g)��H����1627.2 kJ��mol��1�Ƿ��ȷ�Ӧ������ƽ�������ƶ����¶�����ʱ���ᷢ�������Ĵ�������Ӧ����NO2����Ӧ����ʽ��4NH3��7O2 4NO2��6H2O��
4NO2��6H2O��
(3) �ٸ���ͼ3����֪�����ڴ����������£�C2H4��NO��O2��Ӧ��������N2��CO2��H2O����ϩ��NO�������Ϊ1:3�����ݵ�ʧ�����غ㣬��Ӧ�ܷ���ʽΪ6NO+3O2+ 2C2H4![]() 3N2+4CO2+4H2O��
3N2+4CO2+4H2O��
����ͼ����֪����b���ߵ���ߵ㴦�������ʸ��������ʵͣ����˵�����ΪԼΪ350 ����������3.0%��
(4) A.�����������Ӧ���ʼӿ죬ƽ�ⲻ�ƶ���N2Ũ�Ȳ���������A�����ܣ�
B.�����¶ȣ�ƽ�������ƶ���N2Ũ������Ӧ���ʼ�����B���ܣ�
C.����CO2������ƽ�������ƶ���N2Ũ�ȼ�С��C�����ܣ�
D.��С���������˲��N2Ũ������D�����ܣ�
��ѡB��
(5)A����Ӧ��c��ʱ���������ֵ�����������С�����DZ��ֲ��䣬��c��ʱ��Ӧδ��ƽ��״̬��ѡ��A����
B����ʼʱͨ��SO2��NO2����Ӧ������Ӧ����ʼ����b�㷴Ӧ���Ũ�ȴ���c�㣬ѡ��B����
C����c��֮ǰ����Ӧ���Ũ����С���������ݻ����ֲ��䣬����������˵����Ӧ���¶������ߣ��÷�ӦΪ���ȷ�Ӧ����Ӧ����������������������������ѡ��C����
D���������仯���߿�֪��a��b�ε�����Ӧ����С��b��c�ε�����Ӧ���ʣ���t1����t2ʱ��a��b������SO2�����ʵ���С��b��c�Σ���a��b��SO2��ת����С��b��c�Σ�ѡ��D��ȷ��
��ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��һ�������£�2SO2(g)��O2(g)![]() 2SO3(g)(����Ӧ����)����÷�Ӧ������������£�
2SO3(g)(����Ӧ����)����÷�Ӧ������������£�
����1 | ����2 | ����3 | |
��Ӧ�¶�T/K | 700 | 700 | 800 |
��Ӧ��Ͷ���� | 2 mol SO2�� 1 mol O2 | 4 mol SO3 | 2 mol SO2�� 1 mol O2 |
ƽ������(SO2)/mol��L��1��s��1 | v1 | v2 | v3 |
ƽ��c(SO3)/mol��L��1 | c1 | c2 | c3 |
ƽ����ϵ��ѹǿp/Pa | p1 | p2 | p3 |
���ʵ�ƽ��ת������ | ��1(SO2) | ��2(SO3) | ��3(SO2) |
ƽ�ⳣ��K | K1 | K2 | K3 |
����˵����ȷ����
A.��1����3����1(SO2)����3(SO2)
B.K1��K3��p2��2p3
C.c2��2c3����2(SO3)����3(SO2)>1
D.��1����2��c2��2c1
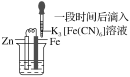
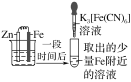
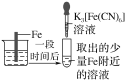
����Ŀ����֤����������������������ʵ������(�ձ��ھ�Ϊ�����ữ��3%NaCl��Һ)��
�� | �� | �� |
|
|
|
��Fe����������ɫ���� | �Թ��������Ա仯 | �Թ���������ɫ���� |
����˵������ȷ���ǣ� ��
A.�ԱȢڢۣ������ж�Zn������Fe
B.��Zn����Cu���âٵķ������ж�Fe��Cu����
C.��֤Zn����Feʱ�����âٵķ���
D.�ԱȢ٢ڣ�K3[Fe(CN)6]���ܽ�Fe����
����Ŀ��ʵ������Ҫ0.1mol/LNaOH��Һ450mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⡣
��1������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_________������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ���������__________��

��2��������ƿ��ʹ�÷����У����в�������ȷ����____________
A��ʹ������ƿǰ�����Ƿ�©ˮ |
B������ƿ��ˮϴ�������ô�����Һϴ�� |
C��������Һʱ����������ǹ��壬�ѳƺõĹ�����ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ� |
D��������Һʱ����������Һ�壬����Ͳȡ�����ò�����������������ƿ�У�������ˮ���̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ� |
E���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ�
��3�����ݼ�����������ƽ��ȡ������Ϊ______g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��_____0.1mol/L��������������С������������������
��4�����ݼ����֪��������Ͳ��ȡ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ��������Ϊ_______mL�����ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ��______mL��Ͳ��á�