��Ŀ����
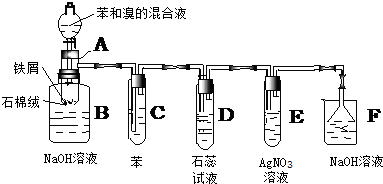
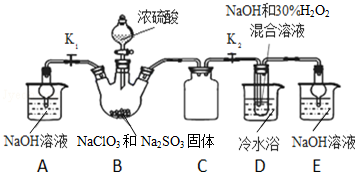
7���������ȣ�ClO2������ɫ������ˮ�����壩�Ǹ�Ч���Ͷ���������������⣺��1��ʵ������NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ�ϣ�ͨ�����¹��̣�ͼ1���Ʊ�ClO2��

�ٵ��ʱ������Ӧ�Ļ�ѧ����ʽΪNH4Cl+2HCl$\frac{\underline{\;ͨ��\;}}{\;}$NCl3+3H2����
����ҺX�д������ڵ���������Cl-��OH-��
�۳�ȥClO2�е�NH3��ѡ�õ��Լ���c�����ţ���
a��ˮ b����ʯ�� C��Ũ���� d������ʳ��ˮ
��2����ͼ2װ�ÿ��Բⶨ�������ClO2�ĺ�����
������ƿ�м��������ĵ⻯�أ���50mLˮ�ܽ���ټ��� 3mL ϡ���
���ڲ���Һ��װ���м���ˮ��ʹҺ��û������Һ��ܵĹܿڣ�
��һ�����Ļ������ͨ����ƿ�����գ�
����������Һ��װ���е�ˮ������ƿ�У�
������0.1000mol•L-1��������Ʊ���Һ�ζ���ƿ�е���Һ
��I2+2S2O32-=2I-+S4O62-����ָʾ����ʾ�յ�ʱ����ȥ20.00mL�����������Һ���ڴ˹����У�
����ƿ��ClO2��⻯�ط�Ӧ�����ӷ���ʽΪ2ClO2+10I-+8H+�T2Cl-+5I2+4H2O��
�ڲ���Һ��װ�õ����������ղ�����ClO2���壨�������ݳ�����
��V�м����ָʾ��ͨ��Ϊ������Һ���ζ����յ����������Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
�ܲ�û������ClO2������Ϊ0.02700 g�������ԭ������Cl 35.5 O 16��
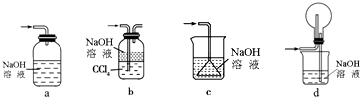
��3����ClO2������������ˮ�Ậ��һ������������Σ���Ҫ��ȥ������������Σ��������������˵���d�����ţ���
a������ b���⻯��c������ d������������
���� �����ڷ�����ClO2�Ļ����ϣ�̽��ʵ������NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ�ϣ�ͨ�����¹����Ʊ�ClO2���漰�Ȼ������������Һ�������NCl3��ԭ����NCl3��NaClO2��Ӧ��ClO2��ԭ�����Լ���Ӧ��Ļ����������ᴿ��������������õζ�ԭ���ⶨ���������ClO2�ĺ����Ļ�������Ҫ�㼰���ݴ�����
��1�����������̿�֪�Ȼ����������Һ�н��е�⣬����������������������NCl3����ⷽ��ʽΪNH4Cl+2HCl$\frac{\underline{\;ͨ��\;}}{\;}$NCl3+3H2������NCl3��Һ�м���NaClO2��������ClO2��NH3��X��X�к�Cl-��OH-������Ϣ��֪��ClO2������ˮ�����Բ�������ˮ��Һ���գ�����Ϊ�������壬�������ʲ�������ᴿ��
��2��������Ŀ��Ϣ��֪��ClO2ͨ����ƿ�����Ե⻯����Һ��Ӧ������I-ΪI2����������ԭΪCl-��ͬʱ����ˮ��
�ڲ���Һ��װ�ÿɷ�ֹ�к������ݳ���
�۵������ⵥ�ʱ�����
�ܸ��ݹ�ϵʽ2ClO2��5I2��10Na2S2O3����n��ClO2�����ٸ���m=nM����m��ClO2����
��3���������ξ��������ԣ�Fe2+��ClO2-��ԭ��Cl-��Fe2+ ������Ϊ�����ӣ�
��� �⣺��1�������������̿�֪�Ȼ����������Һ�е�⣬����������������������NCl3����ⷽ��ʽΪNH4Cl+2HCl$\frac{\underline{\;ͨ��\;}}{\;}$NCl3+3H2����
�ʴ�Ϊ��NH4Cl+2HCl$\frac{\underline{\;ͨ��\;}}{\;}$NCl3+3H2����
����NCl3��Һ�м���NaClO2��������ClO2��NH3��X������NCl3+NaClO2+H2O��ClO2+NH3+NaOH+NaCl����ҺX�д������ڵ���������Cl-��OH-��
�ʴ�Ϊ��Cl-��OH-��
��a��ClO2������ˮ����������ˮ���հ����ʴ���
b����ʯ�Ҳ������հ������ʴ���
c��Ũ����������հ������Ҳ�Ӱ��ClO2������ȷ��
d��ClO2������ˮ���������ñ���ʳ��ˮ���հ������ʴ���
�ʴ�Ϊ��c��
��2��������Ŀ��Ϣ��֪��ClO2ͨ����ƿ�����Ե⻯����Һ��Ӧ������I-ΪI2����������ԭΪCl-��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ2ClO2+10I-+8H+�T2Cl-+5I2+4H2O��
�ʴ�Ϊ��2ClO2+10I-+8H+�T2Cl-+5I2+4H2O��
�ڲ���Һ��װ�õ����������ղ�����ClO2���壨�������ݳ������ʴ�Ϊ�����ղ�����ClO2���壨�������ݳ�����
��V�м����ָʾ��ͨ��Ϊ������Һ���ζ����յ����������Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ���ʴ�Ϊ��������Һ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
�ܺ���Na2S2O3���ʵ���Ϊ0.02 L��0.1mol/L=0.002 mol����
���ݹ�ϵʽ��2ClO2��5I2��10Na2S2O3��
2 10
n��ClO2�� 0.002mol
����n��ClO2��=0.0004mol��
����m��ClO2��=0.004 mol��67.5g/mol=0.02700g��
�ʴ�Ϊ��0.02700��
��3����Ҫ��ȥ������������Σ�ac�����ܻ�ԭ�������Σ�b��KI���л�ԭ�Ե��������ﲻ�ʺ�����ˮʹ�ã�ֻ��d��Fe2+��ClO2-��ԭ��Cl-��Fe2+ ������Ϊ�����ӣ���������ˮ�����ɽ���ɾ�������ˮ���������˵���d��
��ѡd��
���� ���⿼�����ʺ����IJⶨ��Ϊ��Ƶ���㣬Ϊ2015��߿����⣬�������ʵ����ʡ��Ʊ����̡������ķ�ӦΪ���Ĺؼ������ط�����ʵ�顢�����������ۺϿ��飬�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ̼̼˫��������ת����ϩ��һ����˳���칹 | |
| B�� | CH2Cl2��ͬ���칹�����֤��CH4����������Ľṹ | |
| C�� | �ڶ��ױ�ֻ��һ�ֽṹ����֤������������˫���Ľ���ṹ | |
| D�� | ��ϩ����Ȳ��ƽ���ͷ��ӿ���֪CH3-CH=C��CH3��-C��C-CH3���������е�̼ԭ�ӹ��� |