��Ŀ����
10������8��Ԫ�ص����ʡ��������±����У��������ڵڶ����������| Ԫ������ ��� | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 0.89 | 0.82 | 0.77 | 0.99 | 0.75 | 1.17 | 1.43 |
| �����ͻ��ϼ� | +2 | +3 | +4 | +7 | +5 | +4 | +3 | |
| -2 | -4 | -1 | -3 | -4 |
��1���ڵ�Ԫ�ط�����Be���۵�Ԫ���������𣻢���Ԫ�����ڱ��е�λ���ǣ����ڣ��壩�ڶ����ڢ�A�壻������ӽṹʾ��ͼ��

��2���ȽϢݺ͢�����������Ӧˮ��������Դ�С��HClO4��HNO3��
��3���ȽϢ͢ߵ��⻯����ȶ��ԣ�NH3��SiH4�����⻯�����ʽΪ
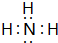 ������������ˮ�����Һ�ʼ��ԣ���ᡱ��������С�����
������������ˮ�����Һ�ʼ��ԣ���ᡱ��������С�������4��д��������������Ӧˮ����������⻯�ﷴӦ�Ļ�ѧ����ʽ��NH3+HNO3=NH4HNO3
��5��д���������������Ӧˮ������������м�����ǿ���������ﷴӦ�����ӷ���ʽAl��OH��3+OH-=AlO2-+2H2O��
��6���ܵ��⻯����ݵĵ��ʣ������Ϊ1��2���ڹ��������·�Ӧ�����ɵ��л�������4�֣����г��������������CH3Cl������4����ͬ���Լ�����CCl4��
���� ��û����ۡ�ֻ����ͼ�-2������֪��ΪO���ۢ�����+3�����ڢ�A�壬ԭ�Ӱ뾶�ۣ��࣬�ʢ�ΪB����ΪAl���ܢ߶������+4����ͼ�-4�����ڢ�A�壬�Ңܵ�ԭ�Ӱ뾶��С���ʢ�ΪC����ΪSi���������+7����ͼ�-1�����ΪCl����ֻ�������+2�����ڢ�A�壬ԭ�Ӱ뾶С��Cl���ʢ�ΪBe���������+5����ͼ�-3�����ڢ�A�壬ԭ�Ӱ뾶С��Cl���ʢ�ΪN���ݴ˽��
��� �⣺��û����ۡ�ֻ����ͼ�-2������֪��ΪO���ۢ�����+3�����ڢ�A�壬ԭ�Ӱ뾶�ۣ��࣬�ʢ�ΪB����ΪAl���ܢ߶������+4����ͼ�-4�����ڢ�A�壬�Ңܵ�ԭ�Ӱ뾶��С���ʢ�ΪC����ΪSi���������+7����ͼ�-1�����ΪCl����ֻ�������+2�����ڢ�A�壬ԭ�Ӱ뾶С��Cl���ʢ�ΪBe���������+5����ͼ�-3�����ڢ�A�壬ԭ�Ӱ뾶С��Cl���ʢ�ΪN��
��1���ڵ�Ԫ�ط�����Be���۵�Ԫ�������ǣ��𣻢�ΪOԪ�أ���Ԫ�����ڱ��е�λ���ǣ��ڶ����ڢ�A�壬�������ΪAl3+�����ӽṹʾ��ͼ�� ��
��
�ʴ�Ϊ��Be���𣻵ڶ����ڢ�A�壻 ��
��
��2���ݺ͢�����������Ӧˮ����ֱ�ΪHClO4��HNO3�������Դ�С��HClO4��HNO3���ʴ�Ϊ��HClO4��HNO3��
��3���͢ߵ��⻯��ֱ�ΪNH3��SiH4���ǽ�����N��Si�����⻯����ȶ��ԣ�NH3��SiH4��NH3���⻯�����ʽΪ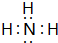 ������������ˮ�����Һ�ʼ��ԣ�
������������ˮ�����Һ�ʼ��ԣ�
�ʴ�Ϊ��NH3��SiH4��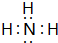 ���
���
��4��������������Ӧˮ����ΪHNO3�������⻯��ΪNH3�����߷�Ӧ�Ļ�ѧ����ʽ��NH3+HNO3=NH4HNO3���ʴ�Ϊ��NH3+HNO3=NH4HNO3��
��5���������������Ӧˮ����ΪAl��OH��3���������м�����ǿ����������ΪNaOH�����߷�Ӧ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��6���ܵ��⻯��ΪCH4����ݵĵ���Cl2�������Ϊ1��2���ڹ��������·�Ӧ�����ɵ��л������У�һ�ȼ��顢���ȼ��顢���ȼ��顢���ȼ���4���л�����г��������������CH3Cl������4����ͬ���Լ����� CCl4��
�ʴ�Ϊ��4��CH3Cl��CCl4��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��Ѷ��еȣ��ؼ��Ǹ��ݻ��ϼ���ԭ�Ӱ뾶�ƶ�Ԫ�أ�ע��Ԫ�������ɵ��������գ�

| ѡ�� | ����ʽ | ������ | ��Ŀ |
| A | C2H4O2 | ����Na2CO3��Һ��Ӧ | 2 |
| B | C4H8Cl2 | ��������һ���� | 3 |
| C | C4H10O | �������Ʒ�Ӧ | 2 |
| D | C8H10 | ����� | 3 |
| A�� | A | B�� | B | C�� | C | D�� | D |

��֪�������������������������ʽ��ȫ����ʱ��pH���±���ʾ���ش����м���
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Ni��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
��2��Ϊ���������ʣ��ɲ�ȡ�Ĵ�ʩ�У������������ϴ���������ʵ�����������Ũ�ȡ�����ʱ���¶ȣ�
��3����A�м��� H2O2ʱ��Ӧ�����ӷ���ʽΪ2Fe2++2H++H2O2=2Fe3++2H2O���Լ�x������NaOH��
��4���÷���ʽ��ʾ���ɳ�������ȡ�������ķ���Ni��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$NiO+H2O��2Al+2NiO$\frac{\underline{\;����\;}}{\;}$Al2O3+3Ni��
����mkg�����ϴ����Ĺ����У�������ҺpH����Ϊ6ʱ������a kgNi��OH��2����ϴ�ӵ��õ���ҺB�Ĺ�����������ʧ��Ϊ3%��������������������ʧ��Ϊ5%�������յõ�������������Ϊ��70%��97%m+$\frac{59}{93}$a����95%kg�������ʽ����
��5�����û�ѧ�ƣ����Ƽ�ֱ�����ں��жƲ�����Ļ��������Һ�У������ڽ��������ϡ��մɵ���Ʒ�������һ�����������Ƚ�����ij��ѧ��������Һ�к���Ni2+��H2PO2-�������������·����ķ�Ӧ֮һ���£�����ƽ�÷�Ӧ��2Ni2++1H2PO2-+H2O=2Ni++1H2PO3-+2H+
������ȣ���ѧ�Ƶ�����ŵ��ǣ������ĵ��ܣ���Լ��Դ��
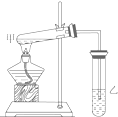 ��ͼ��ijѧ����Ƶ���ȡ����������ʵ��װ��ͼ��ʵ���в�ȡ��������Ҫʵ�������
��ͼ��ijѧ����Ƶ���ȡ����������ʵ��װ��ͼ��ʵ���в�ȡ��������Ҫʵ�������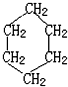 ��
�� C60��N60Ϊ�·��ֵ���������;�ĵ��ʡ����ӽṹ����ͼ��ʾ���о�������C60��N60���������о��ﵽ8�����ȶ��ṹ��
C60��N60Ϊ�·��ֵ���������;�ĵ��ʡ����ӽṹ����ͼ��ʾ���о�������C60��N60���������о��ﵽ8�����ȶ��ṹ��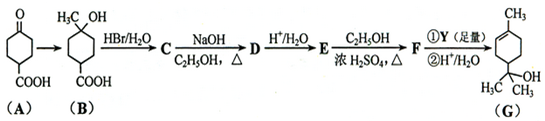
 ���ٺ˴Ź���������2�����շ� ���ܷ���������Ӧ
���ٺ˴Ź���������2�����շ� ���ܷ���������Ӧ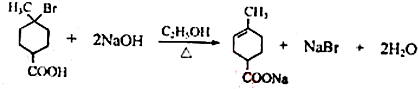 ��
��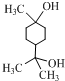 ��
��