ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΔώ.Τϊ≥ΒΈ≤Τχ÷–Κ§”–NOΓΔCOΒ»”–ΚΠΈο÷ Θ§Τδ÷–NOxΜα“ΐΤπΙβΜ·―ß―ΧΈμΒ»ΜΖΨ≥Έ ΧβΓΘ
NH3-SCRΦΦ θ «»Ξ≥ΐNOxΉνΈΣ”––ßΒΡΦΦ θ÷°“ΜΘΚ‘Ύ¥ΏΜ·ΦΝΧθΦΰœ¬Θ§“‘NH3ΜρΡρΥΊΫΪΈ≤Τχ÷–NOxΜΙ‘≠ΈΣN2¥”ΕχΫΒΒΆΈέ»ΨΓΘ
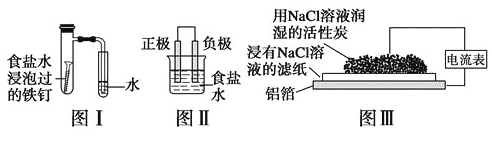
Θ®1Θ©Τϊ≥Β»ΦΝœ÷–“ΜΑψ≤ΜΚ§ΒΣ‘ΣΥΊΘ§ΤϊΗΉ÷–…ζ≥…NOΒΡ‘≠“ρ _________________Θ®”ΟΜ·―ßΖΫ≥Χ Ϋ±μ ΨΘ§ΗΟΖ¥”ΠΈΣΈΣΩ…ΡφΖ¥”ΠΘ©ΘΜΤϊ≥ΒΤτΕ·ΚσΘ§ΤϊΗΉΡΎΈ¬Ε»‘ΫΗΏΘ§ΒΞΈΜ ±ΦδΡΎNO≈≈Ζ≈ΝΩ‘Ϋ¥σΘ§ ‘Ζ÷ΈωΤδ‘≠“ρ _____________________________ΓΘ
Θ®2Θ©ΔΌNH3»Ξ≥ΐΈ≤Τχ÷–ΒΡNOxΘ§Β±v(NO)ΘΚv(NO2)=1:1 ±≥ΤΈΣΓΑΩλΥΌSCR Ζ¥”ΠΓ±Θ§ΗΟΖ¥”ΠΜ·―ßΖΫ≥Χ ΫΈΣ______________________________________ ΘΜ
ΔΎΚœ≥…NH3Υυ”Ο‘≠ΝœΤχH2Θ§Ω…”ΟΧλ»ΜΤχΈΣ‘≠Νœ÷ΤΒΟΘ§”–ΙΊΖ¥”ΠΡήΝΩ±δΜ·»γœ¬Υυ ΨΓΘ
CO(g)+![]() O2 (g)== CO2 (g) ΓςH1=Θ≠282.0 KJ/mol
O2 (g)== CO2 (g) ΓςH1=Θ≠282.0 KJ/mol
H2(g)+![]() O2 (g)=== H2O (g) ΓςH2=Θ≠241.8 KJ/mol
O2 (g)=== H2O (g) ΓςH2=Θ≠241.8 KJ/mol
CH4(g)+ 2O2 (g)== CO2 (g)+ 2H2O (g) ΓςH3=Θ≠836.3 KJ/mol
‘ρ”ΟCH4(g)ΚΆH2O(g)Ζ¥”Π÷ΤΒΟH2(g)ΚΆCO(g)ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ___________ΓΘ
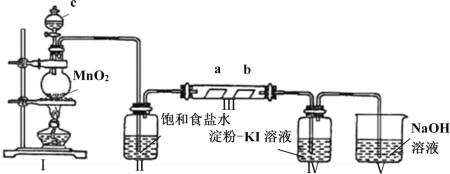
Θ®3Θ©Ά®ΙΐNOx¥ΪΗ–ΤςΩ…Φύ≤βNOxΒΡΚ§ΝΩΘ§ΤδΙΛΉς‘≠άμ»γΆΦΥυ ΨΘ§‘ρΘΚ
ΔΌPtΒγΦΪ…œΖΔ…ζΒΡ « ______________Ζ¥”Π(ΧνΓΑ―θΜ·Γ±ΜρΓΑΜΙ‘≠Γ±)ΘΜ
ΔΎNiOΒγΦΪ…œΒΡΒγΦΪΖ¥”Π ΫΈΣ______________________________________ΘΜ
Θ®4Θ©―–ΨΩΖΔœ÷Θ§ΫΪΟΚΧΩ‘ΎO2/CO2ΒΡΤχΖ’œ¬»Φ…’Θ§ΡήΙΜΫΒΒΆ»ΦΟΚ ±NOΒΡ≈≈Ζ≈Θ§÷ς“ΣΖ¥”ΠΈΣ:2NO(g)+2CO(g)=N2(g)+2CO2(g)ΓΘ‘Ύ“ΜΕ®Έ¬Ε»œ¬Θ§”Ύ2LΒΡΚψ»ίΟή±’»ίΤς÷–≥δ»κ0.1molNOΚΆ0.3molCOΖΔ…ζΗΟΖ¥”ΠΘ§≤βΒΟ≤ΜΆ§ ±Φδ»ίΤςΡΎΒΡ―Ι«Ω(p)”κΤπ Φ―Ι«Ω(p0)ΒΡ±»÷Β(p/p0)»γœ¬±μΓΘ
±Φδ/t | 0min | 2min | 5min | 10min | 13min | 15min |
±»÷Β(p/p0) | 1 | 0.97 | 0.925 | 0.90 | 0.90 | 0.90 |
0ΓΪ5minΡΎΘ§ΗΟΖ¥”ΠΒΡΤΫΨυΖ¥”ΠΥΌ¬ V(NO)=___________________ΘΜ
Θ®5Θ©ΫΪ…œ ωΖ¥”ΠΒΡCO2”κNH3ΈΣ‘≠ΝœΚœ≥…ΡρΥΊΘ§ΡήΙΜ Βœ÷ΫΎΡήΦθ≈≈ΘΚΔΌ2NH3(g)+CO2(g)=NH2CO2NH4span>(s)ΘΜΔΎNH2CO2NH4(s)![]() CO(NH2)2(s)+H2O(g)
CO(NH2)2(s)+H2O(g)
Ε‘”Ύ…œ ωΖ¥”ΠΔΎ‘ΎΟή±’»ίΤς÷–ΫΪΙΐΝΩNH2CO2NH4ΙΧΧε”Ύ300Kœ¬Ζ÷ΫβΘ§ΤΫΚβ ±P[H2O(g)]ΈΣaPaΘ§»τΖ¥”ΠΈ¬Ε»≤Μ±δΘ§ΫΪΧεœΒΒΡΧεΜΐ‘ωΦ”50%Θ§÷Ν¥ο–¬ΤΫΚβΒΡΙΐ≥Χ÷–P[H2O(g)]ΒΡ»Γ÷ΒΖΕΈß «__________________ (”ΟΚ§aΒΡ ΫΉ”±μ Ψ)ΓΘ
ΓΨ¥πΑΗΓΩN2+O2![]() 2NOΈ¬Ε»…ΐΗΏΘ§Ζ¥”ΠΥΌ¬ Φ”Ωλ2NH3+NO+NO2= 2N2+3H2OCH4(g)+H2O(g)== CO(g)+3H2(g) ΓςH=+171.1KJ/molΜΙ‘≠NO-2e-+O2-=NO20.006mol/(LΓΛmin)
2NOΈ¬Ε»…ΐΗΏΘ§Ζ¥”ΠΥΌ¬ Φ”Ωλ2NH3+NO+NO2= 2N2+3H2OCH4(g)+H2O(g)== CO(g)+3H2(g) ΓςH=+171.1KJ/molΜΙ‘≠NO-2e-+O2-=NO20.006mol/(LΓΛmin)![]() aΓήP[H2O]<a
aΓήP[H2O]<a
ΓΨΫβΈωΓΩ
Θ®1Θ©ΒΣ”κ―θΤχ‘ΎΗΏΈ¬œ¬Ζ¥”Π…ζ≥…NOΘ§Ζ¥”ΠΖΫ≥Χ ΫΈΣΘΚN2+O2![]() 2NOΘΜΈ¬Ε»…ΐΗΏΘ§Ζ¥”ΠΥΌ¬ Φ”ΩλΘ§ΒΞΈΜ ±ΦδΡΎNO≈≈Ζ≈ΝΩ‘Ϋ¥σΘ§Ι ¥πΑΗΈΣΘΚN2+O2
2NOΘΜΈ¬Ε»…ΐΗΏΘ§Ζ¥”ΠΥΌ¬ Φ”ΩλΘ§ΒΞΈΜ ±ΦδΡΎNO≈≈Ζ≈ΝΩ‘Ϋ¥σΘ§Ι ¥πΑΗΈΣΘΚN2+O2![]() 2NOΘΜΈ¬Ε»…ΐΗΏΘ§Ζ¥”ΠΥΌ¬ Φ”ΩλΘΜ
2NOΘΜΈ¬Ε»…ΐΗΏΘ§Ζ¥”ΠΥΌ¬ Φ”ΩλΘΜ
Θ®2Θ©ΔΌ”…―θΜ·ΜΙ‘≠Ζ¥”ΠΒΡΙφ¬…Ω…÷ΣΖ¥”ΠΒΡΖΫ≥Χ ΫΈΣΘΚ2NH3+NO+NO2= 2N2+3H2OΘ§Ι ¥πΑΗΈΣΘΚ2NH3+NO+NO2= 2N2+3H2OΘΜΔΎCH4(g)ΚΆH2O(g)Ζ¥”Π÷ΤΒΟH2(g)ΚΆCO(g)ΒΡΖΫ≥Χ ΫΈΣCH4(g)+H2O(g)== CO(g)+3H2(g)Θ§”…Η«ΥΙΕ®¬…Ω…ΒΟΤδΖ¥”ΠΒΡλ ±δΓςH=ΓςH3-3ΓςH2-ΓςH1=+171.1KJ/molΘ§Ι ¥πΑΗΈΣΘΚCH4(g)+H2O(g)== CO(g)+3H2(g) ΓςH=+171.1KJ/molΘΜ
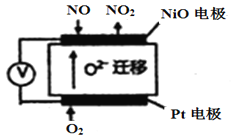
Θ®3Θ©”…ΧβΖΔ…ζΜΙ‘≠Ζ¥”ΠΘ§ΒγΦΪΖ¥”Π ΫΈΣO2+ 4e-=2O2ΓΣΘ§NiOΒγΦΪΈΣ‘≠Βγ≥ΊΒΡΗΚΦΪΘ§ΖΔ…ζ―θΜ·Ζ¥”ΠΘ§ΒγΦΪΖ¥”Π ΫΈΣNO+2e-+O2-= NO2ΓΘΙ ¥πΑΗΈΣΘΚΜΙ‘≠ΓΔNO-2e-+O2-=NO2ΘΜ
Θ®4Θ©”…±μΗώ ΐΨίΩ…÷ΣΘ§5min ±Θ§p/p0=0.925Θ§ΗυΨίΑΔΖϋΌΛΒ¬¬όΕ®¬…ΆΤ¬έΘ§Ά§Έ¬ΚΆΆ§ΧεΜΐ ±p/p0=n/n0=0.925Θ§
(0.4x)Γ¬0.40=0.925Θ§x=0.03molvΘ®NOΘ©=![]() = 0.006mol/Θ®LminΘ©ΘΜΙ ¥πΑΗΈΣΘΚ0.006mol/Θ®LminΘ©ΘΜ
= 0.006mol/Θ®LminΘ©ΘΜΙ ¥πΑΗΈΣΘΚ0.006mol/Θ®LminΘ©ΘΜ
Θ®5Θ©ΫΪΧεœΒΒΡΧεΜΐ‘ωΦ”50%Θ§‘ρP[H2O(g)]±δΈΣ![]() PaΘ§«“ΤΫΚβœρ’ΐœρ“ΤΕ·Θ§Ήν÷’H2OΒΡΤΫΚβ≈®Ε»”κ‘≠ΤΫΚβœύΆ§Θ§‘Ύ–¬ΤΫΚβΧθΦΰœ¬P[H2O(g)]”÷Β»”ΎaPaΘ§Ι
PaΘ§«“ΤΫΚβœρ’ΐœρ“ΤΕ·Θ§Ήν÷’H2OΒΡΤΫΚβ≈®Ε»”κ‘≠ΤΫΚβœύΆ§Θ§‘Ύ–¬ΤΫΚβΧθΦΰœ¬P[H2O(g)]”÷Β»”ΎaPaΘ§Ι ![]() PaΘΦP[H2O(g)]ΓήaPaΘ§Ι ¥πΑΗΈΣΘΚ
PaΘΦP[H2O(g)]ΓήaPaΘ§Ι ¥πΑΗΈΣΘΚ![]() aPaΓήP[H2O]<aPa
aPaΓήP[H2O]<aPa