��Ŀ����
����Ŀ����Դ�������������������ᷢչ������ء�
��1��һ���¶��£��������ݻ���Ϊ2L���ܱ������У��ֱ�����Ӧ��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)��H=-49.0kJ��mol-1������������£�
CH3OH(g)��H2O(g)��H=-49.0kJ��mol-1������������£�
���� | �� | �� |
��Ӧ��Ͷ���� | 1molCO2(g)��3molH2(g) | 1molCH3OH(g����1molH2O(g) |
ƽ��ʱc(CH3OH) | c1 | c2 |
ƽ��ʱ�����仯 | �ų�29.4kJ | ����akJ |
�����������˵���÷�Ӧһ���ﵽƽ��״̬����_________������ĸ����
a��v(CO2)����=v(CH3OH)���� b��������ܶȲ�����ʱ��ı�
c��CO2��CH3OH��Ũ��֮�Ȳ�����ʱ��ı� d�������ƽ����Է�������������ʱ��ı�
�������������䣬�ﵽƽ������в������H2ת���ʵIJ�����_________������ĸ����
a�������¶� b����������H2 c���Ƴ��״� d�������������
��cl_________c2(����>������<������=��)��a=_________��
�ܸ��¶��·�Ӧ��ƽ�ⳣ��K=_________�������з�Ӧ10sʱ�ﵽƽ�⣬��0��10s�ڼ��е�ƽ����Ӧ����v(H2)=_________��
��2����֪��Ӧ��2NO2(����ɫ)![]() N2O4(��ɫ)��H��0����һ������NO2����ע�����к��ڣ���ͼ���������ѹ��ע�����Ĺ���������������ʱ��ı仯��������ɫԽ�����ԽС��������˵����ȷ����__________������ĸ����
N2O4(��ɫ)��H��0����һ������NO2����ע�����к��ڣ���ͼ���������ѹ��ע�����Ĺ���������������ʱ��ı仯��������ɫԽ�����ԽС��������˵����ȷ����__________������ĸ����
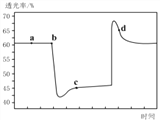
A��b��IJ�����ѹ��ע����
B��d�㣺v����v��
C��c����a����ȣ�c(NO2)����c(N2O4)��С
D������������ϵ�¶ȱ仯����û��������ʧ����Tb��Tc
���𰸡� cd bd = 19.6 2.1��25/12 0.09 mol��L-1��s-1 B
��������(1)��CO2(g)+3H2(g)CH3OH(g)+H2O(g)��H=-49.0kJmol-1��a����Ӧ����֮�ȵ��ڻ�ѧ����ʽ������֮���� v(CO2)����=v(CH3OH)����ֻ��˵����Ӧ������У�����˵����Ӧ�ﵽƽ��״̬����a����b����Ӧǰ�������������䣬������䣬�����ܶ�ʼ�ղ��䣬������ܶȲ�����ʱ��ı䣬����˵����Ӧ�ﵽƽ��״̬����b����c��CO2��CH3OH��Ũ��֮�Ȳ�����ʱ��ı䣬˵�����淴Ӧ������ͬ��˵����Ӧ�ﵽƽ��״̬����c��ȷ��d�������������䣬�������ʵ�����С�������ƽ����Է�������������ʱ��ı䣬˵����Ӧ�ﵽƽ��״̬����d��ȷ���ʴ�Ϊ��cd��
��a����ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ��������У�����ת��������a�����ϣ�b����������H2 ����߶�����̼��ת���ʣ�����ת���ʼ�С����b���ϣ�c���Ƴ��״�ƽ��������У�����ת��������c�����ϣ�d���������������ѹǿ��С��ƽ�������ƶ�������ת���ʼ�С����d���ϣ��ʴ�Ϊ��bd��
��Ӧ�ú��º��������µ��ҵ�Ͷ��������ת��Ϊ���൱��Ͷ1molCO2(g)��3molH2(g)�����������ǵ�Чƽ�⣬����c1=c2���ס����ǵ�Чƽ�⣬����зų����������������յ�����֮��Ϊ49.0kJ����a=49.0kJ-29.4kJ=19.6kJ���ʴ�Ϊ��=��19.6��
�� CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g)��H=-49.0kJmol-1��
CH3OH(g)+H2O(g)��H=-49.0kJmol-1��
��ʼ��(mol) 13 0 0 49KJ
�仯��(mol/L)0.6 1.8 0.6 0.6 29.4KJ
ƽ����(mol/L)0.4 1.2 0.6 0.6
ƽ��Ũ��c(CO2)=0.2mol/L��c(H2)=0.6mol/L��c(CH3OH)=0.3mol/L��c(H2O)=0.3mol/L
K=![]() =
=![]() ��2.1�����з�Ӧ10sʱ�ﵽƽ�⣬��0��10s�ڼ��е�ƽ����Ӧ����v(H2)=
��2.1�����з�Ӧ10sʱ�ﵽƽ�⣬��0��10s�ڼ��е�ƽ����Ӧ����v(H2)= 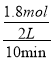 =0.09molL-1s-1���ʴ�Ϊ��
=0.09molL-1s-1���ʴ�Ϊ�� ![]() (��2.1)��0.09molL-1s-1��
(��2.1)��0.09molL-1s-1��
(2)A.b�㿪ʼ��ѹ��ע�����Ĺ��̣�������ɫ������ʱ�С����A����B.c���Ĺյ�������ע�����Ĺ��̣�d����ƽ���������������������ƶ����̣�����v(��)��v(��)����B��ȷ��C.c����ѹ��ע����������������������������������Ũ�ȶ�����C����D.b�㿪ʼ��ѹ��ע�����Ĺ��̣�ƽ�������ƶ�����Ӧ���ȣ�����T(b)��T(c)����D���ʴ�Ϊ��B��