��Ŀ����
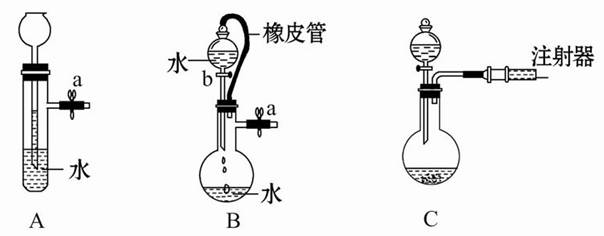
��10�֣���1������������ѧ��ѧʵ���г��õ�����������ʵ������У�һ�㲻��Ҫ�ò��������� ����д��ţ�
�ٴ����ᴿ
������һ�����ʵ���Ũ�ȵ��Ȼ�����Һ
�۽������Ȼ���������Һ�����ˮ���Ʊ�������������
��̽��Ba��OH��2��8H2O�����NH4Cl���巴Ӧ�����е������仯
��ʵ���������Ƶ�FeSO4��Һ��Ԥ��������NaOH��Һ�Ʊ�Fe��OH��2��ɫ����
��2���ٶ�ȡ�ζ����е������ʱ������Һ�棬��ȡ������� ʵ����������>������=����<����
����������ƽ��ȡ10.4gʳ�Σ��������ʳ�ε�λ�õߵ�������ȡʳ�ε�����Ϊ g��
��ʵ������480mL 0.1mol��L-1NaOH��Һ��������Һʱ�����ý�ͷ�ιܡ��ձ���������ƽ�������룩����������ҩ���⣬�������õ��������� ������ȡNaOH���������Ϊ g��
��1���ۢ� ��2���� �� �� 9.6 �� 500ml����ƿ 2.0
���������������1���������ǻ�ѧʵ���г��õ������������������ڽ��衢�������ٴ����ᴿ�IJ����������ܽ⡢���ˡ��������ܽ��ò�������������ܽ⣬�������ò������������������ò����������ֹҺ���оֲ����ȣ���Ҫ��������һ�����ʵ���Ũ�ȵ��Ȼ�����Һ���ܽ�ʱ�õ��ձ������������������������ǽ��裬���ٹ����ܽ⣬������ƿ��ת��Һ���ò�������������Ҫ���۽������Ȼ���������Һ�����ˮ���Ʊ������������壬Ϊ��ֹ����۳������ò��������裬����Ҫ����̽��Ba��OH��2?8H2O�����NH4Cl���巴Ӧ�����е������仯�����ò��������������dz�ֻ�ϣ��ӿ췴Ӧ���ʣ���Ҫ����ʵ���������Ƶ�FeSO4��Һ��Ԥ��������NaOH��Һ�Ʊ�Fe��OH��2��ɫ�������÷�Ӧ���ʿ죬Ϊ��ֹ���������������������ò��������裬����Ҫ��ѡ�ۢݣ���2���ٵζ��ܵ���̶������棬Խ���¿̶�Խ������Һ��������ƫ�������Ŀ̶ȴ���ʵ�ʵĿ̶ȴڸ������̵�����=���̵�����+���������������ҩƷ����=��������-����������ijͬѧ��������ƽ����ʳ��ʱ��ҩƷ������ŵߵ��ƵõĽ����10.4g��1g���������룩����ʳ�ε�ʵ������Ϊ10.0g-0.4g=9.6g������һ���ݻ�������ƿֻ��������Ӧ�������Һ����������ƿ�Ĺ��ѡ��ʵ����û��480ml����ƿ��Ӧѡ��500ml����ƿ��ʵ��������Һ500ml��������500ml 0.1mol/L��NaOH��Һ����Ҫ�������Ƶ����ʵ���Ϊ��0.5��0.1mol/L=0.05mol������Ҫ�������Ƶ�����Ϊ0.05mol��40g/mol=2.0g��
���㣺���黯ѧʵ�����������

����ʵ���ܴﵽĿ�ĵ���
| A����CCl4��ȡ��ˮ�еĵ� |
| B����������������������CaCO3���ʵ�Na2SO4�пɳ�ȥ���� |
| C������������HCl��Cl2ͨ��NaOH��Һ�г�ȥHCl |
| D����Fe(OH)3��������ˮ���Ʊ�Fe(OH)3���� |
ijͬѧ��ʵ�鱨���м�¼�������ݣ���ȷ���� ( )
| A����25ml��Ͳ��ȡ12��36 ml ���� |
| B����������ƽ��ȡ8��75��ʳ�� |
| C���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��ȥNaOH��Һ23��10ml |
| D����pH��ֽ���ij��Һ��PHΪ3��5 |
������������ȷ���ǣ� ��
| A����Ũ���ᱣ������ɫ����ƿ�� |
| B������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ֧�ܿ� |
| C�����Ⱥ���Թܲ�����������ˮ��ϴ����ֹ����ը�� |
| D����������ʱ�����������Ȧ��ֱ�Ӽ��ȣ���������ǯ��ȡ |
����a��e����ѧ��ѧʵ���г����ļ��ֶ���������
(a)��Ͳ (b)����ƿ (c)�ζ��� (d)������ƽ (e)�¶ȼ�
��1���ޡ�0���̶ȵ��� (��д���)��
��2�����в����������� (����ĸ)
| A����25mL��ʽ�ζ�����ȡ20.00mLNaHCO3 |
| B����������ƽȷ����10.20��̼���ƹ��� |
| C����100mL��Ͳ��ȡ3.2mLŨ���� |
| D������1 mol��L�C1������������Һ475mLѡ��500mL����ƿ |

��3��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ������ͼ��ʾ��������������Һ�����Ϊ mL��
��4��ͼ�ױ�ʾ10 mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1 mL������̶�AΪ4����Ͳ��Һ������Ϊ mL��ͼ�ұ�ʾ25 mL��ʽ�ζ�����ij��������D��E ֮��Ŀ̶Ȳ�Ϊ1 mL������̶�DΪ4�������ʽ�ζ�����Һ������Ķ���Ϊ mL��
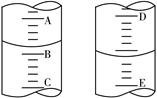
ͼ�� ͼ��