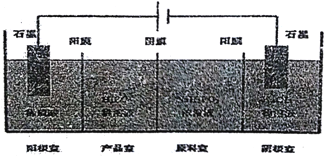
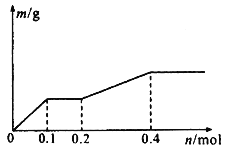
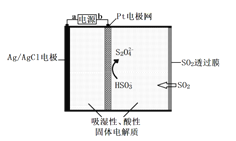
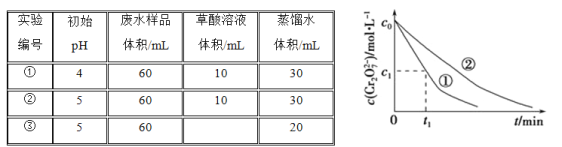
��Ŀ����
����Ŀ������X��Y��Z����Ԫ�أ���֪�����������
��X��Y��Z�ĵ����ڳ����¾�Ϊ���塣
�� X�ĵ�����Z�ĵ�����ȼ�գ�����XZ��ȼ��ʱ����ʲ�ɫ��
�� XZ��������ˮ����ˮ��Һ�е����X����Z����XZ��ˮ��Һ��ʹʯ����Һ��졣
��������X�ĵ��ʿ���һ����Y�ĵ��ʻ�������������X2Y��X2Y������ΪҺ�塣
�� Z�ĵ�������X2Y�У�������Һ����Ư�����á�
������������⣺
��1��д��XZ��X2Y�Ļ�ѧʽ��XZ X2Y
��2��Z�ĵ�������X2Y����Һ��Ư�����õ������� ��д��ѧʽ����
��3��д��ʵ������ȡX�ĵ��ʵ���������ʽ _______________��
��4��Z�ĵ����ܷ�������������Һ������Ӧ�� ����ܡ��������ܣ���д����Ӧ����������ʽ�� _______________��
�������ܷ�Ӧ����˿ղ�����д����
��������1�� HCl H2O (2)HClO (3)MnO2+ 4H++2Cl- ![]() Mn2+ +Cl2��+2H2O
Mn2+ +Cl2��+2H2O
��4���� Cl2 +2OH-��Cl- +ClO- +H2O
��������
�������������X��Y��Z�ĵ����ڳ����¾�Ϊ���壬��X�ĵ�����Z�ĵ�����ȼ�գ�����XZ��ȼ��ʱ����ʲ�ɫ����XZΪHCl����XZ��������ˮ����ˮ��Һ�е����X+��Z-��XZ��ˮ��Һ��ʹʯ����Һ��죬����XΪH2��ZΪCl2������������X�ĵ��ʿ���һ����Y�ĵ��ʻ�������������X2Y��X2Y������ΪҺ�壬X2YΪH2O������Z�ĵ�������X2Y�У�������Һ����Ư��������������ˮ��Ӧ����HClO����Һ����Ư���ԣ�
��1���������Ϸ�����֪XZ��X2Y�Ļ�ѧʽ�ֱ���HCl��H2O��
��2��������ˮ��Ӧ����HClO����Һ����Ư���ԣ�
��3��ʵ�����Ʊ����������ӷ���ʽΪMnO2+ 4H++2Cl- ![]() Mn2+ +Cl2��+2H2O��
Mn2+ +Cl2��+2H2O��
��4��Z�ĵ�������������������Һ������Ӧ�����ӷ���ʽΪCl2 +2OH-��Cl- +ClO- +H2O��