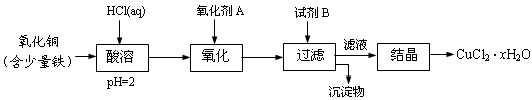
��Ŀ����
��8�֣�ijѧ���������ͼ��װ�ã��ⶨ2 mol/L��������п����п�۷�Ӧ�����ʡ���ش� 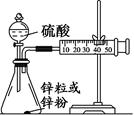
ͼ�� ͼ��
��1��װ��ͼ���з������������������ ��
��2������ͼ��װ��ʵ��ʱ������������ʵ��ʱ���Ϊ10 min��������Ҫ�ⶨ����һ�������� ��
��3��ʵ������õ��Ľ����� ��
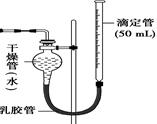
��4�������ֽ�ͼ��װ���е������ռ�װ�ø�Ϊͼ��ʵ����ϴ���ȴ��������ȡ�ζ�����Һ�����ڴ��Ŀ̶��������ֵζ�����Һ����ڸ������Һ�棬Ӧ���Ȳ�ȡ�IJ����� ��


ͼ�� ͼ��
��1��װ��ͼ���з������������������ ��
��2������ͼ��װ��ʵ��ʱ������������ʵ��ʱ���Ϊ10 min��������Ҫ�ⶨ����һ�������� ��
��3��ʵ������õ��Ľ����� ��
��4�������ֽ�ͼ��װ���е������ռ�װ�ø�Ϊͼ��ʵ����ϴ���ȴ��������ȡ�ζ�����Һ�����ڴ��Ŀ̶��������ֵζ�����Һ����ڸ������Һ�棬Ӧ���Ȳ�ȡ�IJ����� ��
��8�֣� ��1����Һ©�� ��2���ռ�����������
��3������������ͬʱ��п�۱�п����Ӧ�����ʿ� ��4�����ڵζ��ܵĸ߶�ʹ������Һ����ƽ
��3������������ͬʱ��п�۱�п����Ӧ�����ʿ� ��4�����ڵζ��ܵĸ߶�ʹ������Һ����ƽ
�����������1������װ�õ��ص��֪��װ��ͼ���з�����������������Ƿ�Һ©����
��2��Ҫ�ⶨ��Ӧ���ʣ�����Ҫ�ⶨ����һ�������ռ�������������
��3����������Ӧ��ĽӴ���������Լӿ췴Ӧ���ʣ����Ը�ʵ���еó��Ľ���������������ͬʱ��п�۱�п����Ӧ�����ʿ졣
��4����������������ѹǿӰ��������ڶ���֮ǰ����Ҫ��ȡ�Ĵ�ʩ�ǵ��ڵζ��ܵĸ߶�ʹ������Һ����ƽ��
�������������е��Ѷȵ����⣬���������ǿ��ע�ض�ѧ������֪ʶ�Ĺ��̺�ѵ���������ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԡ�����������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�
�����Ŀ
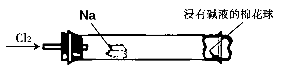

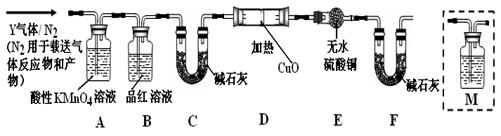

 Na2SO4 + SO2��+ H2O���ݴ��������ͼ��ʾװ�ý������ʵ�顣
Na2SO4 + SO2��+ H2O���ݴ��������ͼ��ʾװ�ý������ʵ�顣 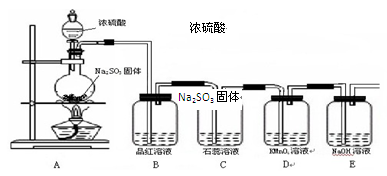
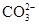 ���μ�ϡ���ᣬ������������ͨ�����ʯ��ˮ
���μ�ϡ���ᣬ������������ͨ�����ʯ��ˮ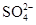 ���ȵμ�ϡ���ᣬ�ٵμ�BaCl2��Һ
���ȵμ�ϡ���ᣬ�ٵμ�BaCl2��Һ