��Ŀ����
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ������������֪��ʵ������ȡSO2����ķ�Ӧԭ��ΪNa2SO3 + H2SO4  Na2SO4 + SO2��+ H2O���ݴ��������ͼ��ʾװ�ý������ʵ�顣
Na2SO4 + SO2��+ H2O���ݴ��������ͼ��ʾװ�ý������ʵ�顣
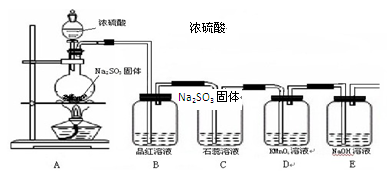
��ش��������⣺
��1��ʢװŨ����������������� ��
��2������ͼ���Ӻ�װ���� ������ ����������ƣ���Ȼ������Լ������ȡ�
��3��ʵ������У� װ��B��C�пɹ۲쵽��ʵ������ֱ��� �� ��
��4��D�пɹ۲쵽��������_______________��˵��SO2���� �ԡ�
��5��װ��E�������� ��
 Na2SO4 + SO2��+ H2O���ݴ��������ͼ��ʾװ�ý������ʵ�顣
Na2SO4 + SO2��+ H2O���ݴ��������ͼ��ʾװ�ý������ʵ�顣 
|
��1��ʢװŨ����������������� ��
��2������ͼ���Ӻ�װ���� ������ ����������ƣ���Ȼ������Լ������ȡ�
��3��ʵ������У� װ��B��C�пɹ۲쵽��ʵ������ֱ��� �� ��
��4��D�пɹ۲쵽��������_______________��˵��SO2���� �ԡ�
��5��װ��E�������� ��
��1����Һ©������2 �֣� ��2�����װ�õ������ԡ���2 �֣�
��3��Ʒ����Һ��ɫ��1�֣���ʯ����Һ��죨1�֣�
��4��KMnO4��Һ��ɫ��1�֣���������ԭ��1�֣�
��5������SO2����ֹ��Ⱦ��������2 �֣�
��3��Ʒ����Һ��ɫ��1�֣���ʯ����Һ��죨1�֣�
��4��KMnO4��Һ��ɫ��1�֣���������ԭ��1�֣�
��5������SO2����ֹ��Ⱦ��������2 �֣�
�����������1�����������Ĺ����ص��֪��ʢװŨ����������������Ƿ�Һ©����
��2��װ�����Ӻ��Ժ�����װ�õ������ԡ�
��3��A������SO2��SO2����Ư���ԣ�������ˮ���������ᣬ��Һ�����ԣ�����װ��B��C�пɹ۲쵽��ʵ������ֱ�Ʒ����Һ��ɫ��ʯ����Һ��졣
��4��SO2�����л�ԭ�ԣ���ʹ���Ը��������Һ��ɫ������D�пɹ۲쵽��������KMnO4��Һ��ɫ��
��5��SO2�Ǵ�����Ⱦ�����װ��E������������SO2����ֹ��Ⱦ������2���Ʊ������ʼ��顢β�������Լ�������ʶ��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ��ע�ػ���֪ʶ�Ĺ�����ѵ������������������������������ѧ���淶���Ͻ���ʵ��������������ѧ�����ۺ�ʵ������������ѧ����ѧ�����������������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
�����Ŀ




 ��
��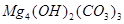 ��
�� �ȡ��������һ���ⶨ��ʽ̼��þ��ɵ�ʵ�鷽��������
�ȡ��������һ���ⶨ��ʽ̼��þ��ɵ�ʵ�鷽��������