��Ŀ����
����Ŀ����1��ij����Һ�п��ܺ��д���Mg2+��Cu2+��Fe3+��K+��H+��NO3-��SO42-��OH-�����е�һ�ֻ��֣���ͨ����ʵ����м��飺
��ȡ��������Һ����ϸ�۲죬����ɫ��
������������Һ�еμ�NaOH��Һ���������������а�ɫ�������ɣ�
����������Һ�м���BaCl2��Һ��������
�ݴ˿����жϸô���Һ��һ���������ڵ�������_______��һ�����ܴ������ڵ�������_______������ȷ���Ƿ���ڵ�������___________��
��2����״���£�H2��CO�Ļ�����干8.96L�����������Ϊ6.0g���Լ���˻��������H2������Ϊ___________��CO���Ϊ________��
���𰸡�Mg2+��H+��NO3- Cu2+��Fe3+��SO42-��OH- K+ 0.4g 4.48L
��������
��ȡ��������Һ����ϸ�۲죬����ɫ������Һ�в�������ɫ���ӣ���Cu2+��Fe3+��
������������Һ�еμ�NaOH��Һ���������������а�ɫ�������ɣ�����Һ�к���H+��Mg2+�������䷴Ӧ�����Ӳ��ܴ��ڣ���OH-��
����������Һ�м���BaCl2��Һ������������Һ�в�����SO42-����Һ�б�Ȼ���������ӣ���ֻ��ΪNO3-��
��1��������֪����Һ�к���Mg2+��H+��NO3-��һ�����ܴ������ڵ�����Cu2+��Fe3+��SO42-��OH-������ȷ���Ƿ����K+��
��2����״���£�H2��CO�Ļ�����干8.96L����0.4mol����ƽ��Ħ������Ϊ![]() =15g/mol����ʮ�ֽ��淨
=15g/mol����ʮ�ֽ��淨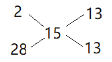 �������ʵ���֮��Ϊ1��1������0.2mol��������������Ϊ0.4g��CO����µ����Ϊ4.48L��
�������ʵ���֮��Ϊ1��1������0.2mol��������������Ϊ0.4g��CO����µ����Ϊ4.48L��

 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�����Ŀ��ijͬѧ��ʵ������ͭ��Ũ���ᷴӦ��ʵ�顣
��1��д����Ӧ�Ļ�ѧ����ʽ_____��
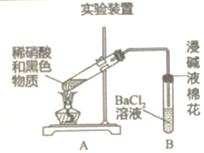
ֹͣ���������Թ��еĻ������ȴ����װ����ˮ���ձ��У����衢���ã��۲쵽�ձ��ײ��к�ɫ���ʡ��������Ժ�ɫ���ʽ�����̽����
��2����ͬѧ�����ɫ����CuO������������£�
���������ף�������Cu2���ķ����ǣ�����Һ�еμ�K4[Fe(CN)6]��Һ�����������ɫ������֤����Cu2������ͬѧ��ʵ�������
�ٽ�CuO����ϡ�����У�һ��ʱ��μ�K4[Fe(CN)6]��Һ���������ɫ������
�ڽ���ɫ���ʷ���ϡ�����У�һ��ʱ��μ�K4[Fe(CN)6]��Һ��δ�����ɫ������ʵ��ٵ�Ŀ����__________���ɸü���������ý�����________��
��3���ٴμ��裬��ɫ������ͭ�����ʵ�����£�
ʵ��װ��
| ���� 1.A�Թ��к�ɫ�������ܽ� 2.A�Թ����Ϸ�����dz����ɫ���� 3.B�Թ��г��֡��� |
������2˵����ɫ���ʾ���________�ԡ�
�� A�Թ����Ϸ�����dz����ɫ����Ļ�ѧ����ʽ��__________��
����ȷ�Ϻ�ɫ�����к���SԪ�ص�����_________��
��4������ʵ��˵������ɫ�����д���ͭ�������һ��ʵ���֤����ɫ������ CuS��Cu2S�Ļ�����֪1molCu2S��ϡ���ᷴӦת��8mole-��д���Թ�A��Cu2S �ܽ�Ļ�ѧ����ʽ____________��