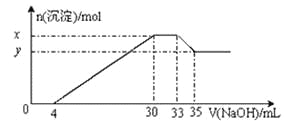
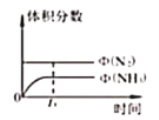
��Ŀ����
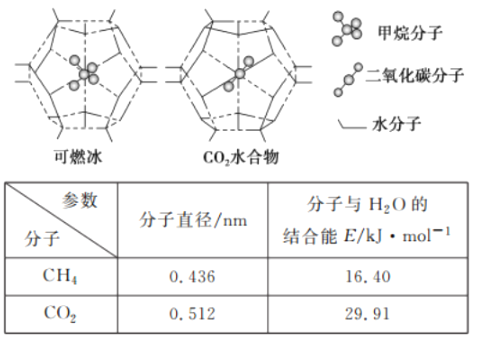
����Ŀ����(Co)�ж��ֻ�����.�ڸ�ѹ������.�����ܵ��ѭ�����Ʊ����������ͼ��ʾ��
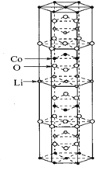
(1)һ����ܸ��������ᄃ��Ľṹ��ͼ��ʾ���仯ѧʽΪ___________��
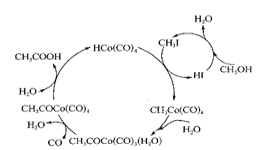
(2)��ͼ��ʾ���ܷ�Ӧ�Ļ�ѧ����ʽΪ______________________��
(3)��̬Coԭ�ӵļ۵����Ų�ͼΪ______________��
(4)1��CH3COCo(CO)4�����к���![]() ������ĿΪ______����CO32����Ϊ�ȵ������һ�ַ��ӵĻ�ѧʽΪ______________��
������ĿΪ______����CO32����Ϊ�ȵ������һ�ַ��ӵĻ�ѧʽΪ______________��
(5)CH4��CO2�Ǻϳ�CH3OH�Ļ���ԭ�ϡ�һ�������£�CH4��CO2������H2O�γ���״�ṹ(����ͼ��ʾ)��ˮ���ᄃ�壬����ز������±��� CH4��H2O�γɵ�ˮ���ᄃ��.�׳�����ȼ������Ϊ�������������ȼ�������п�ѧ���������CO2��CH4�����롣��֪��ͼ����״�ṹ�Ŀ�ǻֱ��Ϊ0.586 nm.���ͼ�������ʽṹ�����ʵĽǶȷ������������������___________��
���𰸡�LiCoO2 CH3OH+CO![]() CH3COOH
CH3COOH ![]() 14 SO3 CO2�ķ���ֱ��С����״��ǻֱ������CO2��H2O�Ľ���ܴ���CH4��H2O�Ľ����
14 SO3 CO2�ķ���ֱ��С����״��ǻֱ������CO2��H2O�Ľ���ܴ���CH4��H2O�Ľ����
��������
��1������ܸ��������ᄃ��Ľṹ��֪��Li�����ھ�����6��λ�����⡢4��λ�������⡢1��λ�����ڡ�2��λ�ڲ����ģ�Li���Ӹ���Ϊ��6��1/3+4��1/4+1+2��1/2=5��, Co�����ھ�����12��λ�ڶ��㡢4��λ���������ġ�4��λ�������⣬Co���Ӹ���Ϊ��12��1/6+4��1/2+4��1/4=5��,O�����ھ�����18��λ�����⡢2��λ�����ڡ�4��λ�ڲ����ģ�Li���Ӹ���Ϊ��18��1/3+2+4��1/2=10��, Li���ӡ�Co���Ӻ�O���Ӹ�����Ϊ5:5:10=1:1:2����ѧʽΪLiCoO2���ʴ�Ϊ��LiCoO2��
��2����ͼ��֪�ұ������ȥ1mol��ˮ���������ֳ���1mol��ˮ������ʵ�ʷ�Ӧ���Ǽ״�����������ȥ1mol��ˮ���������ֳ���1mol��ˮ�����뷴Ӧ����һ����̼�������ɵĴ��ᣬ�����ܷ�Ӧ�ķ���ʽΪ��CO+CH3OH![]() CH3COOH���ʴ�Ϊ��CO+CH3OH
CH3COOH���ʴ�Ϊ��CO+CH3OH![]() CH3COOH��
CH3COOH��
��3��Co��27��Ԫ�أ����������Ϊ27�����������Ų�ʽΪ��1s22s22p63s23p63d74s2���۵����Ų�ʽΪ3d74s2������۵����Ų�ͼΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��4��CH3COCo(CO)4��λ�������У�����ԭ��Co�������γ�5���ļ���CH3CO����5���ļ���4��CO�й���4���ļ�������14�����ļ���CO32����ԭ�Ӹ���Ϊ3���۵�����Ϊ24�������������ͬԭ�Ӹ����ͼ۵������ĵȵ��������ΪSO3���ʴ�Ϊ��SO3��
(5) �ɱ����֪��������̼�ķ���ֱ��С����״�ṹ�Ŀ�ǻֱ������0.512��0.586����˳��������״��ǻ�ڣ��Ҷ�����̼��ˮ�Ľ������ǿ�ڼ��飬��29.91��16.40���ʴ�Ϊ��CO2�ķ���ֱ��С����״��ǻֱ��������H2O�Ľ���ܴ���CH4��

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�����Ŀ�����ᾧõ��������ǿ�ҵ�õ����������һ�ֺܺõĶ�������仯ѧ����Ϊ���������ȼ�����������ͨ�������ȼ������״��ʹ�����Ϊԭ���Ʊ���
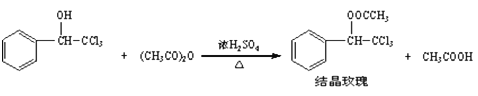
��֪��
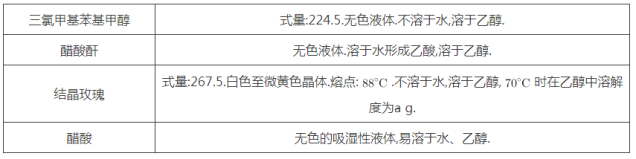
�����������£�
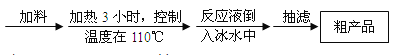
�����������Ϣ���ش��������⣺
(1)����ʱ��Ӧ�ȼ������ȼ������״��Ϳ�������Ȼ����������Ũ���Ტ______������Ͼ��Ⱥ������˵ļ��ȷ�ʽΪ______(����ˮԡ������������ԡ������)��
(2)�ֲ�Ʒ�ijɷ��ǽᾧ����������_______(������)�Ļ�����������·��������ᴿ�ͼ��顣����ɱ������ݣ�
��� | ʵ�鷽�� | ʵ������ | ���� |
�� | ���ֲ�Ʒ�ܽ����У����ֲ�Ʒ���ܼ���������Ϊ1��__��ϣ���ˮԡ���ȵ�70������ܼ�ʹ�ֲ�Ʒ����ܽ� | �õ���ɫ��Һ | |
�� | ������1������Һ____________���� | �õ���ɫ���� | |
�� | ���ﲽ��2���ð�ɫ���壬____________ | _____________ | ��ɫ�����ǽᾧõ�� |
(3)4��51g���ȼ������״���������������ַ�Ӧ�õ��ᾧõ��4.68g���������________(С���������λ��Ч����)��