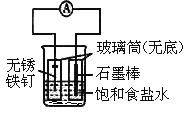
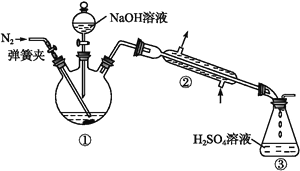
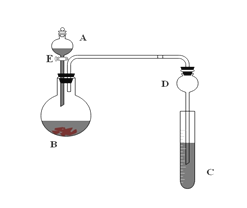
��Ŀ����
����Ŀ���п�Ժ��������������ϸ������ij����ַ�������ͼ��ʾ��

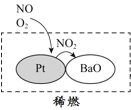
(1)������ͼ��Ϣ���Կ��������е�������Ⱦ�ﲢ���ɻ�������ʻ��ɵ���______����������������ϡȼ����ϵͳ��Ҫ����ԭ��������ͼ��ʾ��д��ϡȼ������NO��������Ҫ��Ӧ�ķ���ʽ_____________________________________��
(2)ũҵ��ų��İ�������ʩ�õĻ��ʷֽ⣬Ҳ������ʩ�ò������µġ�����ijЩ��������Է��ϻ��ʩ�û��ͳ����������ӷ���ʽ����________________________��
(3)�����о������ҹ����������ԣ�����Ҫԭ������ͼ��ʾ��A�Ļ�ѧʽ��________��
2NH3(��)+SO2(��)+2NO2(��)![]() 2NH4+(��Һ)+A(��Һ)+2HONO(��)
2NH4+(��Һ)+A(��Һ)+2HONO(��)
(4)úȼ���ŷŵ���������SO2��NOx������NaClO2��Һ��Ϊ���ռ���ͬʱ��������������������������SO2��NOx������ͨ��ʢ��NaClO2��Һ�ķ�Ӧ���У���Ӧһ��ʱ������Һ������Ũ�ȵ��й���������(�����������Ӻ��Բ���)��
���� | Na+ | SO42�� | NO���� | H+ | Cl |
Ũ��/(mol��L1) | 5.5��103 | 8.5��104 | y | 2.8��104 | 3.5��103 |
��NO��NaClO2��Һ��Ӧ�����ӷ���ʽ��___________________��
�ڱ���y��_______��
(5)��ҵ��������Ҳ�п��ܲ���NOx��Ⱦ����д�����������еĵ�һ�������Ĵ������Ļ�ѧ����ʽ___________________________________��
���𰸡�SO2 2NO+O2=2NO2 NH4++OH-![]() NH3��+H2O SO42- 4NO+3ClO2-+2H2O=4NO3-+3Cl-+4H+��4NO+3ClO2-+4OH-=4NO3-+3Cl-+2H2O y=5.8��104 4NH3+5O2
NH3��+H2O SO42- 4NO+3ClO2-+2H2O=4NO3-+3Cl-+4H+��4NO+3ClO2-+4OH-=4NO3-+3Cl-+2H2O y=5.8��104 4NH3+5O2![]() 4NO+6H2O
4NO+6H2O
��������
(1)����ͼ����Ϣ����֪����������ʻ��ɵ���Ⱦ��NOx��VOCs��NH3��ϡȼ������NO�ᱻ�����������ɶ���������
(2)����������Ȳ��������������
(3)���ݷ�Ӧǰ��Ԫ�ػ��ϼ۵ı仯����ϵ���غ������
(4)��һ��������NaClO2��Һ����������ԭ��Ӧ���������������������ݵ�ʧ�����غ㡢����غ��Լ�ԭ���غ������𣻢ڸ��ݵ���غ���y��ֵ��
(5)���Ĵ���������һ��������ˮ����ԭ���غ㡢�����غ���д��Ӧ����ʽ��
(1)����ͼ����Ϣ����֪����������ʻ��ɵ���Ⱦ��NOx��VOCs��NH3���������������ɻ�������ʻ��ɵģ�ϡȼ������NO�������������ɳɶ�����������Ӧ����ʽΪ��2NO+O2=2NO2��
(2)�̬��������Է��ϻ��ʩ�û��ͷų���������Ӧ�ķ���ʽ��NH4++OH-![]() NH3��+H2O��
NH3��+H2O��
(3)��ӦǰSΪ+4�ۣ�NΪ+4�ۣ���Ӧ��HONO��NԪ�صļ�̬Ϊ+3�ۣ���Ԫ�ػ��ϼ۽��ͣ�����Ԫ�ػ��ϼ����ߣ��ۺ��ƶϣ�Ӧ����+4���ߵ�+6������SO42-������
(4)�ٸ��ݱ����ṩ�����ӿ�֪NO��NaClO2����ΪNO3-��NaClO2����ԭΪCl-������ӦΪ���Ի�������ϵ����غ㡢����غ㣬�ɵ÷�Ӧ�����ӷ���ʽ�ǣ�4NO+3ClO2-+2H2O=4NO3-+3Cl-+4H+������ҺΪ���Ի�����������ӦΪ��4NO+3ClO2-+4OH-=4NO3-+3Cl-+2H2O��
�ڸ�����Һ�ʵ����ԣ�����е�y=5.5��10-3+2.8��10-4-3.5��10-3-2��8.5��10-4=5.8��10-4��
(5)���������е�һ����Ӧ�ǰ��Ĵ���������Pt���������ڼ��������£�NH3��O2������Ӧ������NO��ˮ����Ӧ��ѧ����ʽΪ��4NH3+5O2![]() 4NO+6H2O��
4NO+6H2O��

����Ŀ����1����֪��Ti(s) +2Cl2(g) = TiCl4(l) ��H = ��804.2 kJ ��mol��1��
2Na(s) +Cl2(g) = 2NaCl(s) ��H = ��882.0 kJ ��mol��1
Na(s) = Na(l) ��H =2.6 kJ ��mol��1
��д����Һ̬�������Ȼ����û����ѵ��Ȼ�ѧ����ʽ__________________________��
��2����֪����һ������㶨Ϊ2L���ܱ������г���4mol A��1mol B���������·�Ӧ��4A(g)��B(s) ![]() 3C(s)��4D(g)���÷�Ӧ�и����ʵ�Ħ����������λg��mol��1������һ������һ�������¸÷�Ӧ2���Ӵﵽƽ�⡣
3C(s)��4D(g)���÷�Ӧ�и����ʵ�Ħ����������λg��mol��1������һ������һ�������¸÷�Ӧ2���Ӵﵽƽ�⡣
�ٲ��ܹ�˵���÷�Ӧ�Ѵﵽƽ����ǣ�________��
A�������£������ڵ�ѹǿ���ٱ仯
B�������£������ڻ��������ܶȲ��ٱ仯
C��һ�������£�D������������ֲ���
D��һ�������£���λʱ��������4molA��ͬʱ����1 mol B
��ƽ�����D��Ũ��Ϊ0.3mol��L��1����ӷ�Ӧ��ʼ��ƽ��ʱ��A��ƽ����Ӧ����Ϊ________��Bת����Ϊ________��
��3���û���̿��ԭ�����Դ�����������йط�ӦΪ��2NO(g)+C(s) ![]() N2(g)+CO2(g)����H����ij�ܱ���������һ�����Ļ���̿��NO����t���·�Ӧ���й�������ͼ��
N2(g)+CO2(g)����H����ij�ܱ���������һ�����Ļ���̿��NO����t���·�Ӧ���й�������ͼ��
NO | N2 | CO2 | |
��ʼŨ��/molL��1 | 0.10 | 0 | 0 |
ƽ��Ũ��/molL��1 | 0.04 | 0.03 | 0.03 |
����t���£��÷�Ӧ��ƽ�ⳣ��Ϊ________��������λ��Ч���֣���
��ƽ��������¶ȣ��ٴδﵽƽ�⣬���������NO��N2��CO2��Ũ��֮��Ϊ2:1:1����÷�Ӧ��
��H________0(�����������������������ʱNO��ת����Ϊ________��
����Ŀ����������ԭ�ζ����ⶨij�ֲ��ᾧ����H2C2O4��X H2O���нᾧˮ��������ʵ�鲽�����£�
���÷�����ƽ��ȡ���ᾧ��1.260g���������Ƴ�100.00mL���������Һ��
������Һ����ȡ25.00mL���������Һ����ƿ�У����������������ữ
����Ũ��Ϊ0.1000 mol/L��KMnO4����Һ���еζ������ν�����£�
��һ�εζ� | �ڶ��εζ� | �����εζ� | |
������Һ���(mL) | 25.00 | 25.00 | 25.00 |
����Һ���(mL) | 9.99 | 10.01 | 10.00 |
��֪:H2C2O4����Է�������Ϊ90��
�ش��������⣺
��1���ζ�ʱ��KMnO4����ҺӦ��װ��_____ (������ʽ��������ʽ��)�ζ����С�
��2��H2C2O4����Һ����KMnO4��Һ��Ӧ�Ļ�ѧ����ʽ��______________
��3������ζ��յ�ı�־��____________��
��4�������������ݼ���X=________________��
��5��������(�ƫ�ߡ�ƫ�͡���Ӱ��)��
�����ζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ���Xֵ_________��
�����ζ���ˮϴ��ֱ�Ӽ���KMnO4����Һ����Xֵ_______��