��Ŀ����
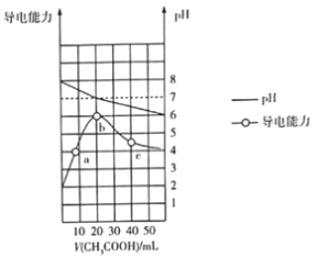
����Ŀ�������£�����ͬŨ�ȵ�KOH��Һ���ֱ�ζ�Ũ�Ⱦ�Ϊ0.1 mol��L1�������ᣨHA��HB��HD����Һ���ζ�������ͼ��ʾ�������ж���ȷ����
A. ���кͰٷ�����100%ʱ����������Һ��Ϻ�c(HA)+c(HB)+c(HD)��c(H+)+c(OH)
B. �ζ���P��ʱ����Һ�У�c(K+)> c(A)> c(HA)> c(H+)> c(OH)
C. ͬŨ�ȵ�KA��KB��KD��������Һ��pH��С��ϵ��pH(KD)< pH(KB)< pH(KA)
D. �������ᶼ�к�������ʱ������KOH��Һ������Ĵ�С��ϵΪ��V(HA) > V(HB) > V(HD)
���𰸡�D
��������
A.���кͰٷ�����100%ʱ�������ʷֱ���KA��KB��KD�����������غ�ɵ�c(HA)��c(HB)��c(HD)��c(OH��)��c(H��)����A����B. �ζ���P��ʱ����Ϊ�����ʵ���Ũ�ȵ�HA��KA����Һ�����ԣ�HB�ĵ���Ϊ����������̶Ƚ�С�����c(A��)��c(K��)��c(HA)��c(H��)��c(OH��)����B����C.��������(HA��HB��HD)��Һ����ʼpH��֪������ĵ��볣����ϵ��KHA��KHB��KHD������Ũ����ͬ�����A-��B-��D-��ˮ��̶�������ǿ��ͬŨ�ȵ�KA��KB��KD��������Һ�ĵļ���Ҳ������ǿ������pH(KD)>pH(KB) > pH(KA)����C����D.��������ĵ��볣��ΪKHA��KHB��KHD���ʵ��������Ũ����ͬʱ��HA��������������Ũ����������ᶼ�к�������ʱ�����ĵ�KOHҲ��࣬��������KOH��Һ������Ĵ�С��ϵΪ��V(HA) > V(HB) > V(HD)����D��ȷ����ѡD��

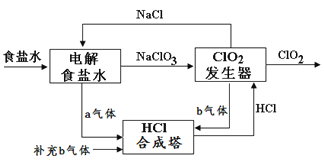
����Ŀ���п�Ժ��������������ϸ������ij����ַ�������ͼ��ʾ��

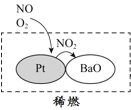
(1)������ͼ��Ϣ���Կ��������е�������Ⱦ�ﲢ���ɻ�������ʻ��ɵ���______����������������ϡȼ����ϵͳ��Ҫ����ԭ��������ͼ��ʾ��д��ϡȼ������NO��������Ҫ��Ӧ�ķ���ʽ_____________________________________��
(2)ũҵ��ų��İ�������ʩ�õĻ��ʷֽ⣬Ҳ������ʩ�ò������µġ�����ijЩ��������Է��ϻ��ʩ�û��ͳ����������ӷ���ʽ����________________________��
(3)�����о������ҹ����������ԣ�����Ҫԭ������ͼ��ʾ��A�Ļ�ѧʽ��________��
2NH3(��)+SO2(��)+2NO2(��)![]() 2NH4+(��Һ)+A(��Һ)+2HONO(��)
2NH4+(��Һ)+A(��Һ)+2HONO(��)
(4)úȼ���ŷŵ���������SO2��NOx������NaClO2��Һ��Ϊ���ռ���ͬʱ��������������������������SO2��NOx������ͨ��ʢ��NaClO2��Һ�ķ�Ӧ���У���Ӧһ��ʱ������Һ������Ũ�ȵ��й���������(�����������Ӻ��Բ���)��
���� | Na+ | SO42�� | NO���� | H+ | Cl |
Ũ��/(mol��L1) | 5.5��103 | 8.5��104 | y | 2.8��104 | 3.5��103 |
��NO��NaClO2��Һ��Ӧ�����ӷ���ʽ��___________________��
�ڱ���y��_______��
(5)��ҵ��������Ҳ�п��ܲ���NOx��Ⱦ����д�����������еĵ�һ�������Ĵ������Ļ�ѧ����ʽ___________________________________��
����Ŀ�������ü�������NO2��Ⱦ�����о���CH4+2NO2![]() N2+CO2+2H2O����1L�ܱ������У����Ʋ�ͬ�¶ȣ��ֱ����0.50molCH4��1.2molNO2�����n(CH4)��ʱ��仯���й�ʵ�����ݼ��±���
N2+CO2+2H2O����1L�ܱ������У����Ʋ�ͬ�¶ȣ��ֱ����0.50molCH4��1.2molNO2�����n(CH4)��ʱ��仯���й�ʵ�����ݼ��±���
��� | �¶� | ʱ��/min n/mol | 0 | 10 | 20 | 40 | 50 |
�� | T1 | n(CH4) | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
�� | T2 | n(CH4) | 0.50 | 0.30 | 0.18 | 0.15 |
����˵����ȷ������ ��
A. ��ʵ�����ݿ�֪ʵ����Ƶ��¶�T2>T1
B. ������У�0~20min�ڣ�NO2�Ľ�������Ϊ0.0125mol��L-1��min-1
C. 40minʱ��������T2��Ӧ������Ϊ0.18
D. 0��10min�ڣ�CH4�Ľ���������>��