��Ŀ����
20��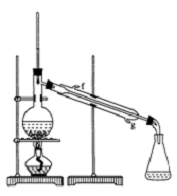 ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��
ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ����֪��
| �ܶ� ��g/cm3�� | �۵� ���棩 | �е� ���棩 | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
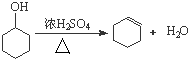
������дA�л������ƻ���ϩ�Ļ�ѧ��Ӧ����ʽ
 ��
����A�����Ƭ�������Ƿ�ֹ���У�����B���˵�������е�������������
���Թ�C���ڱ�ˮԡ�е�Ŀ���Ƿ�ֹ����ϩ�Ļӷ���
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȣ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ���� �㣨��ϡ����¡�������Һ����C�������ţ�ϴ�ӣ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ����ͼװ����������ʱҪ������ʯ�ң�Ŀ���dz�ȥˮ�֣�
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��83�����ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����C��
A������ʱ��70�濪ʼ�ռ���ƷB��������ʵ����������C���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������B��C��
A�������Ը��������ҺB���ý�����C���ⶨ�е㣮
���� ��1���ٴ���Ũ����������·�����������ˮ����ϩ�����������ڷ�������ˮ���ɻ���ϩ��
�ڷ���װ��A�����Ƭ�������Ƿ�ֹ���У��������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ������
�۱�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ����
��2���ٻ���ϩ�������Ȼ�����Һ�����ܶȱ�ˮС���ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ��������ᴿ����ʱ��c��Na2CO3��Һ��ϴ�ӿɳ�ȥ�
����ʯ������ˮ��Ӧ�����������ƣ��ܳ�ȥˮ�֣�
�۸��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棻
A������ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�����
B����ȡ�Ļ���ϩ���ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�����
C���ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�����
��3�����ݻ����û�й̶��ķе㣬���������й̶��ķе㣬�ݴ˿��жϲ�Ʒ�Ĵ��ȣ�
��� �⣺��1���ٻ�������Ũ����������·�������ˮ���ɻ���ϩ����ӦΪ�� ��
��
�ʴ�Ϊ�� ��
��
�ڷ���װ��A�����Ƭ�������Ƿ�ֹ���У��������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ������
�ʴ�Ϊ����ֹ���У�������
�۱�ˮԡ��Ŀ���ǽ��ͻ���ϩ�������¶ȣ�ʹ��Һ����
�ʴ�Ϊ����һ����ȴ����ֹ����ϩ�ӷ���
��2���ٻ���ϩ�����࣬�������Ȼ�����Һ���ܶ�0.81g/cm3���ܶȱ�ˮС�������á��ֲ��ϩ���ϲ㣬���ڷ�Һ��ϩ��Ʒ�л�������������ͻ��������������ᣬҲ���������Ը�����أ��������������ϩ����c��Na2CO3��Һ��ϴ�ӿɳ�ȥ�ᣬ
�ʴ�Ϊ���ϲ㣻c��
����ʯ������ˮ��Ӧ�����������ƣ���ȥ�˲�����ˮ���õ������Ļ���ϩ��
�ʴ�Ϊ����ȥˮ�֣�
�۸��ݱ������ݿ�֪����ֻ���ϩ�ķе�Ϊ83�棬���ռ���ƷӦ�����¶���83�����ң�
A������ʱ��70�濪ʼ�ռ���Ʒ����ǰ�ռ�����Ʒ�л������ʣ�ʵ�ʲ����������۲�������A����
B��������ʵ���������ˣ���ȡ�Ļ���ϩ�����ʵ�������ʵ���ƵõĻ���ϩ��Ʒ�����������۲�������B����
C�����ֲ�Ʒ�л��л����������²ⶨ���ĵĻ������������ƵõĻ���ϩ��Ʒ�����������۲�������C��ȷ��
�ʴ�Ϊ��83�棻C��
��3�������Ʒ�뾫Ʒ�ɼ�������ƣ��۲��Ƿ�������������������壬���Ǿ�Ʒ��������ݻ����û�й̶��ķе㣬���������й̶��ķе㣬ͨ���ⶨ����ϩ��Ʒ�ͻ���ϩ��Ʒ�ķе㣬Ҳ���жϲ�Ʒ�Ĵ��ȣ�
�ʴ�Ϊ��BC��
���� �����Ի���ϩ���Ʊ�Ϊ���壬�ۺϿ������л��ϳɣ�������ѧ������֪ʶ����������Ŀ�Ѷ��еȣ�ע�����ʵ��ԭ���ͷ������ر���ʵ��Ļ���������ѧϰ��ע����ۣ�

| A�� | ����Һ�����ʵ���Ũ�ȴ��ڼ���Һ�����ʵ���Ũ�� | |
| B�� | ����Һ��H+��Ũ��С�ڼ���Һ��0H-��Ũ�� | |
| C�� | ��ͼ���ʱ������� | |
| D�� | ����Һ��pH�����ҺpH֮��Ϊ14 |
 Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ��ķ�������̽����
Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ��ķ�������̽����ʵ��һ����ͬѧ����Al��Fe��Mg��2mol/L��ϡ���ᣬ���ʵ�鷽���о�Ӱ�췴Ӧ���ʵ����أ�
�о���ʵ�鱨�����±���
| ʵ�鲽�� | ���� | ���� |
| �ٷֱ�ȡ�������2mol/L���������Թ��У��ڷֱ�Ͷ���С����״��ͬ��Al��Fe��Mg | ��Ӧ������Mg��Al��Fe | ��Ӧ�������Խ���ã���Ӧ����Խ�� |
Ҫ�ó���ȷ��ʵ����ۣ�������Ƶ�ʵ���������¶���ͬ��
��2����ͬѧΪ�˸���ȷ���о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죬������ͼװ��ͬ���½��ж���ʵ�飬�ô�С��״��ͬ��Fe�ֱ��0.5mol/L��2mol/L������ϡH2SO4��Ӧ��ͨ���ⶨ�ͱȽ�ͬʱ���ڲ����������������˵��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죮
ʵ�������֪2KMnO4+5H2C2O4+3H2SO4�TK2SO4+2MnSO4+8H2O+10CO2�����ڿ�ʼһ��ʱ���ڣ���Ӧ���ʽ�������Һ��ɫ�����ԣ�������ͻȻ��ɫ����Ӧ�������Լӿ죮
��1�������������ijͬѧ��Ϊ�÷�Ӧ���ȣ�������Һ�¶���������Ӧ���ʼӿ죮��Ӱ�컯ѧ��Ӧ���ʵ����ؿ�������뻹������Mn2+�Ĵ����õ�Ӱ�죮
��2������ʵ��֤����IJ��룬�����Ը��������Һ��������Һ�⣬�����ڷ�Ӧһ��ʼʱ����B��
A������� B�������� C���Ȼ��� D��ˮ��
| A�� | K+��Ca2+��Cl-��SO42- | B�� | NH4+��HCO3-��Cl-��K+ | ||
| C�� | Cl-��Na+��NO3-��Ca2+ | D�� | Fe2+��NO3-��I-��Cl- |
 Ϊ���ۺ����õع��ͣ��ҹ����ع���������������Ϳ��滻������ȼ�ϣ�Ŀǰ�����Ѿ��dz����죮��������Ǹ�֬���������������֬��״�ͨ�������������������´��õ�����֪��֬��״��������ܣ���Ӧ����������֬�����ˮ�����������Ľ�������������ӦΪ��R1COOR2+R3OH��R1COOR3+R2OH���ع��͵�Ԥ�����������£�
Ϊ���ۺ����õع��ͣ��ҹ����ع���������������Ϳ��滻������ȼ�ϣ�Ŀǰ�����Ѿ��dz����죮��������Ǹ�֬���������������֬��״�ͨ�������������������´��õ�����֪��֬��״��������ܣ���Ӧ����������֬�����ˮ�����������Ľ�������������ӦΪ��R1COOR2+R3OH��R1COOR3+R2OH���ع��͵�Ԥ�����������£�
 +Br2$\stackrel{Fe}{��}$
+Br2$\stackrel{Fe}{��}$ +HBr
+HBr
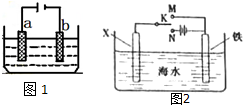 �绯ѧԭ���ڹ�ҵ����������ҪӦ�ã���ͼ1��ʾ�����У�
�绯ѧԭ���ڹ�ҵ����������ҪӦ�ã���ͼ1��ʾ�����У�