��Ŀ����
15���屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�
����1����a�м���15mL����������м���ٽ�b��4.0mLҺ���������뵽a�У���ַ�Ӧ��
����2����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����3����Һ������10mLˮ��8mL 10%��NaOH��Һ��10mL ˮϴ�ӣ���Һ�ô��屽��
����4����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����˼��ôֲ�Ʒ��
��1������1����a�з�������Ҫ��Ӧ��
 +Br2$\stackrel{Fe}{��}$
+Br2$\stackrel{Fe}{��}$ +HBr
+HBr��2������d������������HBr����Ⱦ������
��3����b�е�Һ���������뵽a�У������ܿ��ټ����ԭ���Ƿ�ֹ��Ӧ�ų�����ʹC6H6��Br2�ӷ���Ӱ�����
��4������c��������������������������Ҫ������C6H6��Br2���ѧʽ��
��5������4�õ��Ĵֲ�Ʒ�л��������ʱ�����֪�����屽���й����������������Ҫ��һ���ᴿ�ֲ�Ʒ����������е�ʵ���������������
| �� | �� | �屽 | |
| �ܶ�/g•cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ��ˮ�е��ܽ�� | �� | �� | �� |
���� ��1��ʵ���Һϳ��屽�DZ���Һ���������������������·���ȡ����Ӧ�����屽���廯�⣻
��2����������d�����ջӷ�����HBr��
��3�����ݸ÷�ӦΪ���ȷ�Ӧ��������ķе�ϵ����ӷ����з�����
��4�����ݱ������ݿ�֪������Һ��ķе�ϵͣ����ױ������ӷ�������
��5��������Ĵ��屽�к���δ��Ӧ�ı������뻥�ܵ�Һ�壬���ݷе㲻ͬ����������ķ������з��룮
��� �⣺��1����a�м���15mL����������м���ٽ�b��4.0mLҺ���������뵽a�У���ַ�Ӧ�����屽���廯�⣬��Ӧ�Ļ�ѧ����ʽΪ�� +Br2$\stackrel{Fe}{��}$
+Br2$\stackrel{Fe}{��}$ +HBr��
+HBr��
�ʴ�Ϊ�� +Br2$\stackrel{Fe}{��}$
+Br2$\stackrel{Fe}{��}$ +HBr��
+HBr��
��2������d�з�������Ҫ��Ӧ������HBr���壬����Ⱦ��������
�ʴ�Ϊ������HBr����Ⱦ��������
��3������Һ��ķ�ӦΪ���ȷ�Ӧ��������Һ���ٶȹ��죬��Ӧ��ų��϶�����������ڱ�����ķе�ϵͣ����±������ӷ�������Ӱ�����屽�IJ��ʣ���b�е�Һ���������뵽a�У���ֹ��Ӧ�ų�����ʹC6H6��Br2�ӷ���Ӱ����ʣ�
�ʴ�Ϊ����ֹ��Ӧ�ų�����ʹC6H6��Br2�ӷ���Ӱ����ʣ�
��4�����ķе�Ϊ80�棬��ķе�Ϊ59�棬���߷е�ϵͣ����ӷ�������������������������Ҫ����Ϊ��C6H6��Br2��
�ʴ�Ϊ��C6H6��Br2��
��5�����÷е㲻ͬ�����ķе�С������������屽����ĸҺ�У����Բ�ȡ����ķ��������屽�뱽���ʴ�Ϊ������
���� ���⿼���˱������ʡ��Ʊ�ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ��漰���屽����ȡʵ�顢���ʵķ����ᴿ�ȣ�����Ʊ���ԭ���ǽ��Ĺؼ������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������������

 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�| A�� | ÿ����6.72 L��H2����Һ��AlO2-����Ŀ������0.2NA | |
| B�� | ÿ����0.15 molH2������ԭ��ˮ������ĿΪ0.3NA | |
| C�� | ������2.7gAlʱ��ת�Ƶĵ�����ĿΪ0.3NA | |
| D�� | ��Һ��ÿ����0.1mol��AlO2-��Na+����Ŀ������0.1NA |
�������ķ�����Ŀ֮��Ϊ1��1 ��������Oԭ����Ŀ֮��Ϊ1��2
��������ԭ������Ŀ֮��Ϊ2��3�����ߵ��ܶ�֮��Ϊ��7��11
�������ĵ�����Ŀ֮��Ϊ7��11��
| A�� | �ٺ͢� | B�� | �ں͢� | C�� | �ܺ͢� | D�� | �٢ڢۢܢ� |
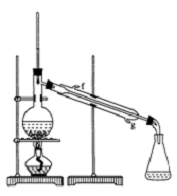
 ����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С��������ͼװ�úϳ�����ȩ��
����ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С��������ͼװ�úϳ�����ȩ�������ķ�Ӧ���£�CH3CH2CH2CH2OH$��_{H_{2}SO_{4}��}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/�� | �ܶ�/g•cm-3 | ˮ���ܽ��� | |
| ������ | 117.2 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
��Na2Cr2O7��Һ��Ũ������Һ������B�У���A�м����������ͼ�����ʯ�����ȣ����ַ�Ӧ�¶�Ϊ90��95�棬��E���ռ�90�����ϵ���֣�
������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77����֣�
�ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����У�˵�����ɲ��ܣ��������Ž���
��2������װ��ͼ�У�B�����������Ƿ�Һ©����D������������ֱ�������ܣ�
��3���¶ȼƵ����÷ֱ���C1C1���Ʒ�Ӧ�¶ȣ�C2C2�ⶨ�������������¶ȣ�
��4��������ȩ�ֲ�Ʒ���ڷ�Һ©����ˮ���²㣨��ϡ����¡�����
��5����Ӧ�¶�Ӧ������90��95�棬��ԭ���Ǽȿɱ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
 ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��
ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ����֪��
| �ܶ� ��g/cm3�� | �۵� ���棩 | �е� ���棩 | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
������дA�л������ƻ���ϩ�Ļ�ѧ��Ӧ����ʽ
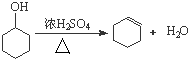 ��
����A�����Ƭ�������Ƿ�ֹ���У�����B���˵�������е�������������
���Թ�C���ڱ�ˮԡ�е�Ŀ���Ƿ�ֹ����ϩ�Ļӷ���
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȣ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ���� �㣨��ϡ����¡�������Һ����C�������ţ�ϴ�ӣ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ����ͼװ����������ʱҪ������ʯ�ң�Ŀ���dz�ȥˮ�֣�
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��83�����ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����C��
A������ʱ��70�濪ʼ�ռ���ƷB��������ʵ����������C���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������B��C��
A�������Ը��������ҺB���ý�����C���ⶨ�е㣮
| A�� | ʹ�ô�����ʹK�仯����H���� | |
| B�� | ʹ�ô�����ʹ��H����K���� | |
| C�� | ʹ�ô���������¶Ⱦ�������H2��ת���� | |
| D�� | ��һ�ܱ������У�Ͷ��Ũ�Ⱦ�Ϊ1mol•L-1�� N2��H2��NH3��ƽ��ǰv��������v���棩 |

 ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�
ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У� ��
��