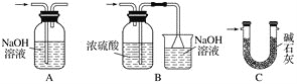
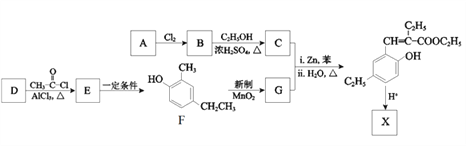
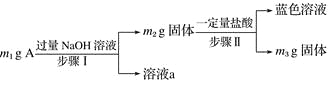
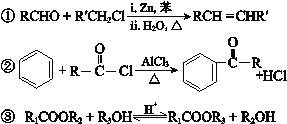��Ŀ����
����Ŀ��һ���ܱ��������м���һ�����ɻ����ĸ���(��Ȳ���)�������ֳ������֣�����߳���8molN2,�ұ߳���CO��CO2�Ļ�����干64gʱ�����崦����ͼλ��(�����¶Ȳ���),����˵����ȷ����(�� ��)

A. �ұ�CO��CO2������֮��Ϊ3:1
B. �Ҳ�CO������Ϊ14g
C. �Ҳ������ܶ�����ͬ�����������ܶȵ�2��
D. ���ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�1/3���������¶Ȳ��䣬��ǰ�����γ�����������ڵ�ѹǿ֮��Ϊ6: 5
���𰸡�A
��������
�������������¶ȡ�ѹǿ��ͬ����ͬ�����£����֮�ȵ������ʵ���֮�ȣ��������֮��Ϊ4:1���������������ʵ���֮��Ϊ4:1�������Ҳ��������ʵ���=8mol/4=2mol��CO��CO2����Ϊ64g����CO�����ʵ���Ϊxmol���������̼���ʵ���Ϊ(2x)mol��28xg+44(2x)g=64g��x=1.5mol����CO�����ʵ���Ϊ1.5mol��������̼�����ʵ���Ϊ0.5mol��
A.��������ʵ���֮�ȵ����������֮�ȣ������ұ�CO��CO2������֮��Ϊ1.5mol:0.5mol=3:1����A��ȷ��
B.m(CO)=nM=1.5mol��28g/mol=42g����B����
C.��ͬ�����������ܶ�֮�ȵ�����Ħ������֮�ȣ��ұ�����ƽ��Ħ������=64g/2mol=32g/mol��������Ħ��������ȣ����Ի�������������ܶ�֮��Ϊ1:1����C����
D.���ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�1/3���������ҿռ����֮��Ϊ2:1�������������ʵ���֮��Ϊ2:1�����������̼��CO���ʵ���Ϊ4mol����ͬ��������������ʵ���֮�ȵ�����ѹǿ֮����������ѹǿ֮��Ϊ(8+2)mol:(8+4)mol=5:6����D����ѡA��

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
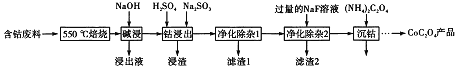
����С��ҵϵ�д�����Ŀ���о�+6�۸��β�ͬ��������������ʽ�������ԣ�ijС��ͬѧ��������ʵ�飺
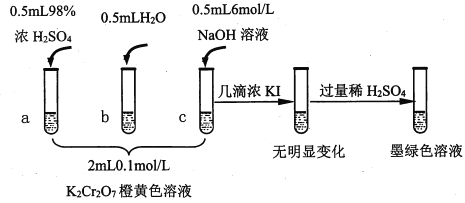
��֪��Cr2O72- (��ɫ)+H2O![]() 2CrO42-(��ɫ)+2H+ ��H= +13.8 kJ/mol��+6�۸�����һ�������¿ɱ���ԭΪCr3+��Cr3+��ˮ��Һ��Ϊ��ɫ��
2CrO42-(��ɫ)+2H+ ��H= +13.8 kJ/mol��+6�۸�����һ�������¿ɱ���ԭΪCr3+��Cr3+��ˮ��Һ��Ϊ��ɫ��
��1���Թ�c��b�Աȣ��Ʋ��Թ�c��������________��
��2���Թ�a��b�Աȣ�a����Һ��ɫ�������Ϊ�¶�Ҳ��Ӱ��ƽ����ƶ�����ɫ���һ����c(H+)����Ӱ��Ľ��������Ϊ��ɫ����һ����c(H+)�����ƽ���Ӱ�졣����Ϊ�Ƿ���Ҫ�����ʵ��֤����____�����ǡ�����������_________________________________��
��3���Ա��Թ�a��b��c��ʵ�����õ��Ľ�����________________��
��4���Թ�c�����μ�KI��Һ������ϡH2SO4��������ͼ��ʵ�����ó��Ľ�����_______��д���˹�����������ԭ��Ӧ�����ӷ���ʽ________________��
��5��С��ͬѧ�õ�ⷨ������Cr2O72-��ˮ��̽����ͬ���ضԺ�Cr2O72-��ˮ������Ӱ�죬������±���ʾ��Cr2O72-����ʼŨ�ȣ��������ѹ�����ʱ�����ͬ����
ʵ�� | �� | �� | �� | �� |
�Ƿ����Fe2(SO4)3 | �� | �� | ����5g | �� |
�Ƿ����H2SO4 | �� | ����1mL | ����1mL | ����1mL |
�缫���� | ����������Ϊʯī | ����������Ϊʯī | ����������Ϊʯī | ����Ϊʯī������Ϊ�� |
Cr2O72-��ȥ����/% | 0.922 | 12.7 | 20.8 | 57.3 |
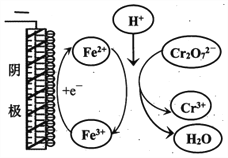
��ʵ������Cr2O72-�ŵ�ĵ缫��Ӧʽ��________________��
��ʵ������Fe3+ȥ��Cr2O72-�Ļ�����ͼ��ʾ����ϴ˻���������ʵ��iv��Cr2O72-ȥ������߽϶��ԭ��_______________��