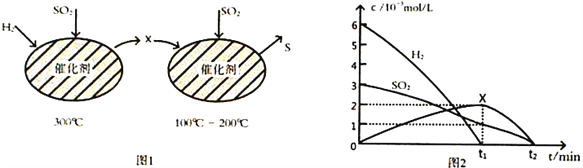
��Ŀ����
����Ŀ���л���F�� ��Ϊһ�ָ߷�����֬����ϳ�·�����£�
��Ϊһ�ָ߷�����֬����ϳ�·�����£�

��֪����AΪ����ȩ��ͬϵ���������������Է�������Ϊ134��
��
��ش��������⣺
��1��X�Ļ�ѧ������_________________��
��2��E����F�ķ�Ӧ����Ϊ_________________��
��3��D�Ľṹ��ʽΪ_________________��
��4����B����C�Ļ�ѧ����ʽΪ_________________��
��5�������廯����Y��D��ͬϵ�Y��ͬ���칹�����뱥��Na2CO3��Һ��Ӧ�ų����壬������ֻ��1���������˴Ź���������ʾ��5�ֲ�ͬ��ѧ�������⣬��ֵ�����Ϊ6:2:2:1:1��д�����ַ���Ҫ���Y�Ľṹ��ʽ___________��__________��
��6��д���Լ�ȩ����ȩ���Ҷ���Ϊ��Ҫԭ�Ϻϳ����������۾��߷��Ӳ��ϡ��ۼ���ϩ���������� ���ĺϳ�·�ߣ����Լ���ѡ����_________________��
���ĺϳ�·�ߣ����Լ���ѡ����_________________��
���𰸡� ��ȩ ���۷�Ӧ 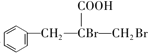
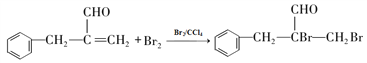
��������������Ϣ��AΪ����ȩ��ͬϵ���������������Է�������Ϊ134��A����ȩ������F��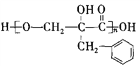 �Ľṹ��ʽ��֪��EΪ�����ǻ��ķ������ᣬ����C�ķ���ʽ������D��E������֪��C��DΪ�ǻ���ȩ����������D��EΪ±��ԭ�ӵ�ˮ�ⷴӦ�����E�Ľṹ��֪��DΪ������ԭ�ӵķ������ᣬ��CΪ������ԭ�ӵķ���ȩ��BΪ����̼̼˫���ķ���ȩ��������Ϣ�ڿ�֪XΪ��ȩ��
�Ľṹ��ʽ��֪��EΪ�����ǻ��ķ������ᣬ����C�ķ���ʽ������D��E������֪��C��DΪ�ǻ���ȩ����������D��EΪ±��ԭ�ӵ�ˮ�ⷴӦ�����E�Ľṹ��֪��DΪ������ԭ�ӵķ������ᣬ��CΪ������ԭ�ӵķ���ȩ��BΪ����̼̼˫���ķ���ȩ��������Ϣ�ڿ�֪XΪ��ȩ��
��1������������, XΪ��ȩ����ȷ�𰸣���ȩ��
��2������F�� ���ṹ��֪�������������ǻ����Ȼ��������۷�Ӧ���ɵģ���˸÷�Ӧ����Ϊ���۷�Ӧ����ȷ�𰸣����۷�Ӧ��
���ṹ��֪�������������ǻ����Ȼ��������۷�Ӧ���ɵģ���˸÷�Ӧ����Ϊ���۷�Ӧ����ȷ�𰸣����۷�Ӧ��
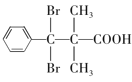
��3������C�ķ���ʽ������D��E������֪��C��DΪ�ǻ���ȩ����������D��EΪ±��ԭ�ӵ�ˮ�ⷴӦ�����E�Ľṹ��֪��DΪ������ԭ�ӵķ������ᣬD�Ľṹ��ʽΪ ����ȷ�𰸣�
����ȷ�𰸣� ��
��
��4�����������֪BΪ����̼̼˫���ķ���ȩ���������巢���ӳɷ�Ӧ����ѧ����ʽΪ��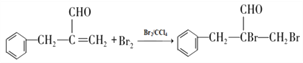 ����ȷ�𰸣�
����ȷ�𰸣�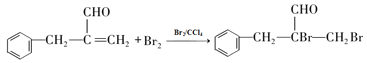 ��
��
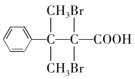
��5��D�Ľṹ��ʽΪ �������廯����Y��D��ͬϵ�˵���������Ȼ�����ԭ�ӣ��ܹ��뱥��Na2CO3��Һ��Ӧ�ų����壬˵�������Ȼ���������ֻ��1���������ṹ�ı仯ֻ��̼���칹����ԭ��λ���칹���˴Ź���������ʾ��5�ֲ�ͬ��ѧ�������⣬��ֵ�����Ϊ6:2:2:1:1��˵����������3����ԭ�ӣ�������Ϊ2:2:1����������2����ԭ�ӣ�������Ϊ6:1������Ҫ���Y�Ľṹ��ʽΪ
�������廯����Y��D��ͬϵ�˵���������Ȼ�����ԭ�ӣ��ܹ��뱥��Na2CO3��Һ��Ӧ�ų����壬˵�������Ȼ���������ֻ��1���������ṹ�ı仯ֻ��̼���칹����ԭ��λ���칹���˴Ź���������ʾ��5�ֲ�ͬ��ѧ�������⣬��ֵ�����Ϊ6:2:2:1:1��˵����������3����ԭ�ӣ�������Ϊ2:2:1����������2����ԭ�ӣ�������Ϊ6:1������Ҫ���Y�Ľṹ��ʽΪ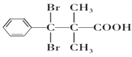 ��
��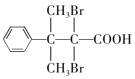 ����ȷ�𰸣�
����ȷ�𰸣�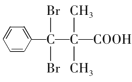 ��
�� ��
��
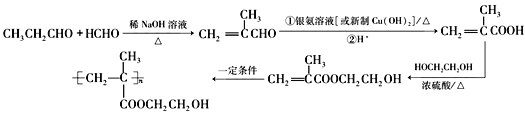
��6��Ҫ�ϳ��л���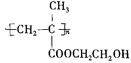 ���͵��Ⱥϳ�CH2=C(CH3)COOCH2CH2OH����Ҫ�ϳ�CH2=C(CH3)COOCH2CH2OH���͵��ü���ϩ�����Ҷ�������������Ӧ��������ϩ��͵��ɼ���ϩȩ��������������ȩ�ͼ�ȩ�ڼ��Ի����·�Ӧ���ɼ���ϩȩ������Ʊ������ʵ��������£�
���͵��Ⱥϳ�CH2=C(CH3)COOCH2CH2OH����Ҫ�ϳ�CH2=C(CH3)COOCH2CH2OH���͵��ü���ϩ�����Ҷ�������������Ӧ��������ϩ��͵��ɼ���ϩȩ��������������ȩ�ͼ�ȩ�ڼ��Ի����·�Ӧ���ɼ���ϩȩ������Ʊ������ʵ��������£�
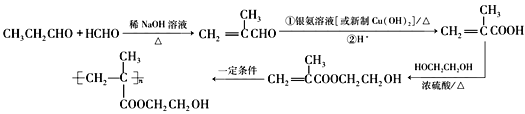 ����ȷ�𰸣�
����ȷ�𰸣�