��Ŀ����
��19�֣��ҹ�������ȼ�ϼ״����ұ�����ʵʩ�����˳���ȼ�ϵ�̼�����Ĵ�Ļ��һЩʡ������½���Ե����ƹ�ʹ�ü״����͡��״���ͨ����ú���������������ɵ�CO��H2��һ�������·������·�Ӧ�Ƶã�CO(g) + 2H2(g)  CH3OH(g)��ͼI��ͼ���ǹ��ڸ÷�Ӧ���������ͼʾ��
CH3OH(g)��ͼI��ͼ���ǹ��ڸ÷�Ӧ���������ͼʾ��

�����ͼʾ�ش��������⣺
��1��ͼI�Ƿ�ӦʱCO��CH3OH��Ũ����ʱ��ı仯������ӷ�Ӧ��ʼ��ƽ�⣬��COŨ�ȱ仯��ʾƽ����Ӧ����v(CO)=_______________��
��2��ͼ���ʾ�÷�Ӧ���й����������ı仯������a��ʾ��ʹ�ô���ʱ��Ӧ�������仯����ͼ���л���ʹ�ô�����������仯����b��
��3��д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��4���÷�Ӧ��ƽ�ⳣ��K�ı���ʽΪ �����¶�����ʱ����ƽ�ⳣ��K��________�����������С�����䡱����
��5�����������£����д�ʩ����ʹ ������� ��
������� ��
A�������¶� B������He��
C���ٳ���1molCO��2molH2 D��ʹ�ô���
��6���ں��������£�����COŨ�Ȳ��䣬���������������ƽ�� ��������ƶ������������ƶ����������ƶ�����
��7�����¶ȡ��ݻ���ͬ�������ܱ������У�����ͬ��ʽͶ�ϣ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й���������
�����й�ϵ��ȷ����
A��c1= c2 B��Q3= 2Q2 C��2 P1��P3
D����1+��2=1 E��2��2=��3
E��2��2=��3
F���÷�Ӧ������1molCH3OH�ų�������Ϊ��Q1+ Q2��kJ
��8������һ����ɱ���ܱ������г���1mol CO��2mol H2��1molCH3OH���ﵽƽ��ʱ��û��������ܶ���ͬ��ͬѹ����ʼ��1.6������÷�Ӧ�� ������������桱����Ӧ�����ƶ���������
 CH3OH(g)��ͼI��ͼ���ǹ��ڸ÷�Ӧ���������ͼʾ��
CH3OH(g)��ͼI��ͼ���ǹ��ڸ÷�Ӧ���������ͼʾ��
�����ͼʾ�ش��������⣺
��1��ͼI�Ƿ�ӦʱCO��CH3OH��Ũ����ʱ��ı仯������ӷ�Ӧ��ʼ��ƽ�⣬��COŨ�ȱ仯��ʾƽ����Ӧ����v(CO)=_______________��
��2��ͼ���ʾ�÷�Ӧ���й����������ı仯������a��ʾ��ʹ�ô���ʱ��Ӧ�������仯����ͼ���л���ʹ�ô�����������仯����b��
��3��д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��4���÷�Ӧ��ƽ�ⳣ��K�ı���ʽΪ �����¶�����ʱ����ƽ�ⳣ��K��________�����������С�����䡱����
��5�����������£����д�ʩ����ʹ
 ������� ��
������� ��A�������¶� B������He��
C���ٳ���1molCO��2molH2 D��ʹ�ô���
��6���ں��������£�����COŨ�Ȳ��䣬���������������ƽ�� ��������ƶ������������ƶ����������ƶ�����
��7�����¶ȡ��ݻ���ͬ�������ܱ������У�����ͬ��ʽͶ�ϣ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й���������
| ���� | �� | �� | �� |
| Ͷ���� | 1mol CO ��2mol H2 | 1molCH3OH | 2molCH3OH |
| CH3OH��Ũ�ȣ�mol��L-1�� | c1 | c2 | c3 |
| ��Ӧ�������仯 | �ų�Q1kJ | ����Q2kJ | ����Q3kJ |
| ��ϵѹǿ��Pa�� | P1 | P2 | P3 |
| ��Ӧ��ת���� | ��1 | ��2 | ��3 |
A��c1= c2 B��Q3= 2Q2 C��2 P1��P3
D����1+��2=1
 E��2��2=��3
E��2��2=��3F���÷�Ӧ������1molCH3OH�ų�������Ϊ��Q1+ Q2��kJ
��8������һ����ɱ���ܱ������г���1mol CO��2mol H2��1molCH3OH���ﵽƽ��ʱ��û��������ܶ���ͬ��ͬѹ����ʼ��1.6������÷�Ӧ�� ������������桱����Ӧ�����ƶ���������
��1��0.075mol/(L��min)
��2��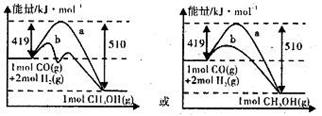
��3��CO(g) + 2H2(g) CH3OH(g)����H= -91kJ��mol-1
CH3OH(g)����H= -91kJ��mol-1
��4��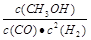 ��С
��С
��5��C
��6�������ƶ� ��7��ADF
��8���� ��Ӧǰ���������������䣬ͬ��ͬѹ�´ﵽƽ��ʱ�����ܶ��������������С��ƽ�������ƶ�
��2��

��3��CO(g) + 2H2(g)
 CH3OH(g)����H= -91kJ��mol-1
CH3OH(g)����H= -91kJ��mol-1��4��
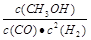 ��С
��С ��5��C
��6�������ƶ� ��7��ADF
��8���� ��Ӧǰ���������������䣬ͬ��ͬѹ�´ﵽƽ��ʱ�����ܶ��������������С��ƽ�������ƶ�
�����������1��v(CO)=
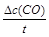 =
= =0.075mol/(L��min)��
=0.075mol/(L��min)����2��ʹ�ô������ͷ�Ӧ��ܣ�����H���䡣ͼ��Ϊ��
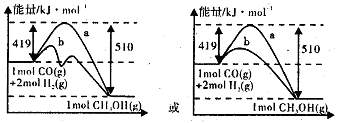
��3����Ӧ�������������������ߣ���ӦΪ���ȷ�Ӧ���Ȼ�ѧ����ʽΪ��
CO��g��+2H2(g)=CH3OH(g) ��H= ��91kJ��mol��1
��4����Ӧƽ�ⳣ������ʽΪ
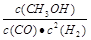 ����ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���K��С��
����ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���K��С����5�������¶ȣ�ƽ�����ƣ�
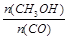 ��С������He����ʹ�ô�����ƽ�ⲻ�ƶ���
��С������He����ʹ�ô�����ƽ�ⲻ�ƶ���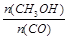 ���䣻�ٳ���1molCO��2molH2��Ч������ѹǿ��ƽ�������ƶ���
���䣻�ٳ���1molCO��2molH2��Ч������ѹǿ��ƽ�������ƶ���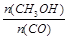 ����
������6�����£�K���䣬����COŨ�Ȳ��䣬�������������Ũ����Q>K��ƽ�����淴Ӧ�����ƶ���
��7��������ȫ��Ч��c1=c2��
 1+
1+ 2=1��Q1+Q2=91��p1=p2��
2=1��Q1+Q2=91��p1=p2��������Ƚϣ�c3>2c2��Q3<2Q2��p3<2p2��
 3<
3< 2��
2����8����ϵ��ȫ�����壬�����غ㣬�ﵽƽ��ʱ�����ܶ��������������С����Ӧ����������С�
�����������ۺ��Խ�ǿ�������ǣ�7����Чƽ����ж��ѶȽϴ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��
O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� �� O2(g)=H2O(l) ��H=��285.84kJ��mol��1
O2(g)=H2O(l) ��H=��285.84kJ��mol��1  H=" -198" kJ��mol-1��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
H=" -198" kJ��mol-1��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
 O2(g)==H2O(l) ��H3= ��285 kJ��mol-1
O2(g)==H2O(l) ��H3= ��285 kJ��mol-1