��Ŀ����
��֪H2(g)�� O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��
O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��
 O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��
O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��| A��H2��ȼ����Ϊ241.8 kJ��mol��1 |
| B��2H2(g)��O2(g)===2H2O(g)����H����483.6 kJ��mol��1 |
| C��1 mol H2��ȫȼ������Һ̬ˮ�ų�����������241.8 kJ |
| D���Ͽ�1 mol H2O�Ļ�ѧ�����յ����������ڶ���1 mol H2��0.5 mol O2�Ļ�ѧ�������յ������� |
A
���������ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų�������������ˮ��״̬����̬�������ȶ�״̬��ˮ���ȶ�״̬Ӧ����Һ̬��A����ȷ��B��ȷ��������̬ˮ����������Һ̬ˮ������������1mol H2��ȫȼ������Һ̬ˮ�ų�����������241.8 kJ��C��ȷ����Ӧ�Ⱦ��Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ������D��ȷ����ѡA��
�������������е��Ѷȵ����⣬Ҳ�Ǹ߿��еij�������֮һ������ע�ػ�����������˫�飬����������ѧ��������������������Ĺؼ�����ȷȼ�����Լ���Ӧ�ȵĸ��Ȼ��������������ü��ɡ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 2C(g)�ﵽƽ��ʱ�������ʵ�
2C(g)�ﵽƽ��ʱ�������ʵ� ����ȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ����֪
����ȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ����֪ ��ȫ����������Ӧ�ų�
��ȫ����������Ӧ�ų� ���������Ȼ�ѧ����ʽ�ǣ�
���������Ȼ�ѧ����ʽ�ǣ� 2NH3(g) ��H����93 kJ��mol��1���Ը��ݱ������м������ݣ�����a ����ֵΪ_______kJ��mol��
2NH3(g) ��H����93 kJ��mol��1���Ը��ݱ������м������ݣ�����a ����ֵΪ_______kJ��mol��
 Al(OH)3(aq)
Al(OH)3(aq) CO+3H2���ʱ��H=_______��
CO+3H2���ʱ��H=_______��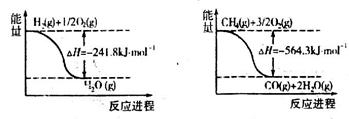
 SO3(g) ��H = �D98.32kJ��mol���������г���2molSO2 ��1molO2��ַ�Ӧ�����շų�������Ϊ
SO3(g) ��H = �D98.32kJ��mol���������г���2molSO2 ��1molO2��ַ�Ӧ�����շų�������Ϊ  CH3OH(g)��ͼI��ͼ���ǹ��ڸ÷�Ӧ���������ͼʾ��
CH3OH(g)��ͼI��ͼ���ǹ��ڸ÷�Ӧ���������ͼʾ��
 ������� ��
������� �� E��2��2=��3
E��2��2=��3