МвДҝДЪИЭ
ЎҫМвДҝЎҝAЎўBЎўCЎўDЎўEҫщОӘУР»ъ»ҜәПОпЈ¬AКЗ·ЦЧУКҪОӘC5H10OөДЦұБҙ»ҜәПОпЈ¬BУлNaHCO3ИЬТәНкИ«·ҙУҰЈ¬ЖдОпЦКөДБҝЦ®ұИОӘ1ЎГ2Ј¬ЛьГЗЦ®јдөД№ШПөИзНјЛщКҫЈЁМбКҫЈәRCH=CHRЎдФЪЛбРФёЯГМЛбјШИЬТәЦР·ҙУҰЙъіЙRCOOHәНRЎдCOOHЈ¬ЖдЦРRәНRЎдОӘНй»щЈ©ЎЈПВБРРрКцХэИ·өДКЗЈЁ Ј©
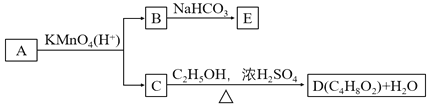
A.BөДҪб№№јтКҪОӘHOOCCH2CH2OHB.DУР6ЦЦН¬·ЦТм№№Ме
C.AҝЙТФУлЗвСх»ҜДЖИЬТә·ўЙъЦРәН·ҙУҰD.EөД·ЦЧУКҪОӘC3H2O4Na2
Ўҫҙр°ёЎҝD
ЎҫҪвОцЎҝ
CәНТТҙјЈ¬ФЪЕЁБтЛбЧцҙЯ»ҜјБөДМхјюПВЈ¬јУИИ·ҙУҰЙъіЙDәНЛ®Ј¬ЛөГч·ўЙъөДКЗхҘ»Ҝ·ҙУҰЈ¬ёщҫЭDЎўЛ®әНТТҙјЦ®јдөД№ШПөЈ¬ҝЙТФөГіцCОӘфИЛбЈ¬ЗТ·ЦЧУКҪОӘC2H4O2Ј¬ДЗГҙCКЗCH3COOHЈ¬DОӘCH3COOCH2CH3Ј¬BДЬУлNaHCO3ИЬТәНкИ«·ҙУҰЈ¬ЗТОпЦКөДБҝЦ®ұИОӘ1ЎГ2Ј¬ЛөГчBОӘфИЛбЈ¬Ҫб№№ЦРҙжФЪБҪёцфИ»щЈ¬ёщҫЭCәНAөД·ЦЧУКҪТФј°ТСЦӘМхјюRCH=CHRЎдФЪЛбРФёЯГМЛбјШИЬТәЦР·ҙУҰЙъіЙRCOOHәНRЎдCOOHЈ¬ҝЙөГіцAөДҪб№№ОӘCH3CH=CHCH2CH2OHЈ¬BОӘHOOCCH2COOHЈ¬EОӘNaOOCCH2COONaЈ¬ҫНҙЛҪшРР·ЦОцЕР¶ПЎЈ
A.УЙ·ЦОцЦӘBОӘHOOCCH2COOHЈ¬AПоҙнОуЈ»
B. DОӘCH3COOCH2CH3Ј¬Н¬·ЦТм№№МеУРхҘАаЎўЛбАаЎўфЗИ©АаЎўфЗНӘАаЎў»·¶юҙјЎўП©¶юҙјөИЈ¬хҘАаУРHCOOCH2CH2CH3ЎўHCOOCH(CH3)2ЎўCH3CH2COOCH3ИэЦЦЈ»ЛбАаУРCH3CH2CH2COOHЎў(CH3)2CHCOOHБҪЦЦЈ¬фЗИ©АаУРHOCH2CH2CH2CHOЎўCH3CH(OH)CH2CHOЎўCH3CH2CH(OH)CHOИэЦЦЈ¬фЗНӘАаУРHOCH2COCH2CH3Ј¬HOCH2CH2COCH3БҪЦЦЈ¬П©¶юҙјУРHOCH2CH=CHCH2OHЈ¬Т»¶Ёі¬№э6ЦЦЈ¬BПоҙнОуЈ»
C. AКфУЪП©ҙјЈ¬І»ДЬәНЗвСх»ҜДЖИЬТә·ўЙъ·ҙУҰЈ¬CПоҙнОуЈ»
D. EОӘNaOOCCH2COONaЈ¬·ЦЧУКҪОӘC3H2O4Na2Ј¬DПоХэИ·Ј»
ҙр°ёСЎDЎЈ

ЎҫМвДҝЎҝYЎўZЎўWЎўRЎўMОеЦЦФӘЛШЈ¬О»УЪФӘЛШЦЬЖЪұнөДЗ°ЛДЦЬЖЪЈ¬ЛьГЗөДәЛөзәЙКэТАҙОФцҙуЈ¬УРИзПВРЕПўЈә
ФӘЛШ | Па№ШРЕПў |
Y | ФӯЧУәЛНвУР6ёцІ»Н¬ФЛ¶ҜЧҙМ¬өДөзЧУ |
Z | ·ЗҪрКфФӘЛШЈ¬»щМ¬ФӯЧУөДs№мөАөДөзЧУЧЬКэУлp№мөАөДөзЧУЧЬКэПаН¬ |
W | ЦчЧеФӘЛШЈ¬УлZФӯЧУөДјЫөзЧУКэПаН¬ |
R | јЫІгөзЧУЕЕІјКҪОӘ3d64s2 |
M | О»УЪөЪўсBЧеЈ¬Ждұ»іЖЧчЎ°өзЖч№ӨТөөДЦчҪЗЎұ |
Зл»ШҙрПВБРОКМв(YЎўZЎўWЎўRЎўMУГЛщ¶ФУҰөДФӘЛШ·ыәЕұнКҫ)Јә
(1)ZЎўWФӘЛШПаұИЈ¬өЪТ»өзАлДЬҪПҙуөДКЗ____Ј¬M2+өДәЛНвөзЧУЕЕІјКҪОӘ_______ЎЈ
(2)M2ZөДИЫөгұИM2WөД______(МоЎ°ёЯЎұ»тЎ°өНЎұ)Ј¬ЗлҪвКНФӯТтЈә_____________ЎЈ
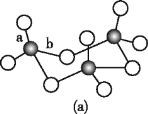
(3)WZ2өДVSEPRДЈРНГыіЖОӘ______Ј»WZ3ЖшМ¬ОӘөҘ·ЦЧУЈ¬ёГ·ЦЧУЦРWФӯЧУөДФУ»Ҝ№мөААаРНОӘ____Ј»WZ3өДИэҫЫМе»·ЧҙҪб№№ИзНј(a)ЛщКҫЈ¬ёГҪб№№өД·ЦЧУЦРә¬УР____ёцҰТјьЈ»РҙіцТ»ЦЦУлWZ3»ҘОӘөИөзЧУМеөД·ЦЧУөД»ҜС§КҪ_____ЎЈ
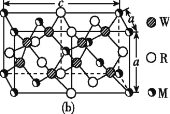
(4)MRW2өДҫ§°ыИзНј(b)ЛщКҫЈ¬ҫ§°ыІОКэa=0.524 nmЎўc=1.032 nmЈ»MRW2өДҫ§°ыЦРЈ¬ҫ§МеГЬ¶ИҰС=____g/cm3(Ц»ТӘЗуБРЛгКҪЈ¬І»ұШјЖЛгіцКэЦөЈ¬°ў·ьјУөВВЮіЈКэОӘNA=6.02ЎБ1023 mol-1)ЎЈ
ЎҫМвДҝЎҝЖыіөОІЖшЦРә¬УРCOәНNOxЈ¬јхЗбЖд¶ФҙуЖшөДОЫИҫіЙОӘҝЖСР№ӨЧчөДИИөгОКМвЎЈ»ШҙрПВБРОКМвЈә
(1)ТСЦӘПВБРИИ»ҜС§·ҪіМКҪЈәCO(g)+2H2(g)![]() CH3OH(g) ҰӨH1Ј¬CO2(g)+3H2(g)=CH3OH(g) +H2O(g) ҰӨH2=-49.0kJЎӨmol-1Ј¬CO(g)+H2O(g)=CO2(g)+H2(g) ҰӨH3=-41.1kJЎӨmol-1ЎЈ
CH3OH(g) ҰӨH1Ј¬CO2(g)+3H2(g)=CH3OH(g) +H2O(g) ҰӨH2=-49.0kJЎӨmol-1Ј¬CO(g)+H2O(g)=CO2(g)+H2(g) ҰӨH3=-41.1kJЎӨmol-1ЎЈ
ФтҰӨH1=_________kJЎӨmol-1ЎЈ
(2)УГ»о»ҜәуөДV2O5ЧчҙЯ»ҜјБЈ¬°ұЖшҝЙҪ«NO»№ФӯіЙN2ЎЈ
ўЩV2O5ДЬёДұд·ҙУҰЛЩВККЗНЁ№эёДұд________КөПЦөДЎЈ
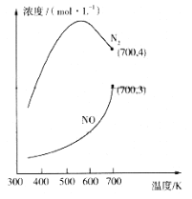
ўЪФЪ1LөДёХРФГЬұХИЭЖчЦР·ЦұрідИл6 mol NOЎў6 mol NH3әНККБҝO2Ј¬ҝШЦЖІ»Н¬ОВ¶ИЈ¬ҫщ·ҙУҰtminЈ¬ІвөГИЭЖчЦРІҝ·Цә¬өӘЖшМеЕЁ¶ИЛжОВ¶ИөДұд»ҜИзНјЛщКҫЎЈNOЕЁ¶ИКјЦХФцҙуөДФӯТтҝЙДЬКЗ______ЎЈ700KКұЈ¬0~tminДЪЈ¬МеПөЦР°ұЖшөДЖҪҫщ·ҙУҰЛЩВКОӘ______ЈЁУГә¬tөДКҪЧУұнКҫЈ©ЎЈ
(3)ҝЖС§јТСРҫҝіцБЛТ»ЦЦёЯР§ҙЯ»ҜјБЈ¬ҝЙТФҪ«COәНNO2БҪХЯЧӘ»ҜОӘОЮОЫИҫЖшМеЈ¬·ҙУҰөДИИ»ҜС§·ҪіМКҪОӘЈә2NO2(g)+4CO(g)![]() 4CO2(g) +N2(g) ҰӨH<0ЎЈДіОВ¶ИПВЈ¬Пт10LәгИЭГЬұХИЭЖчЦРідИл0.1 mol NO2әН0.2 mol COЈ¬·ўЙъЙПКц·ҙУҰЈ¬ЛжЧЕ·ҙУҰөДҪшРРЈ¬ИЭЖчДЪөДС№Зҝұд»ҜИзПВұнЛщКҫЈә
4CO2(g) +N2(g) ҰӨH<0ЎЈДіОВ¶ИПВЈ¬Пт10LәгИЭГЬұХИЭЖчЦРідИл0.1 mol NO2әН0.2 mol COЈ¬·ўЙъЙПКц·ҙУҰЈ¬ЛжЧЕ·ҙУҰөДҪшРРЈ¬ИЭЖчДЪөДС№Зҝұд»ҜИзПВұнЛщКҫЈә
Кұјд/min | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
С№Зҝ/kPa | 75 | 73.4 | 71.96 | 70.7 | 69.7 | 68.75 | 68.75 |
ФЪҙЛОВ¶ИПВЈ¬·ҙУҰөДЖҪәвіЈКэKp =________kPa-1ЈЁKpОӘТФ·ЦС№ұнКҫөДЖҪәвіЈКэЈ©Ј»ИфұЈіЦОВ¶ИІ»ұдЈ¬ФЩҪ«COЎўCO2ЖшМеЕЁ¶И·ЦұрФцјУТ»ұ¶Ј¬ФтЖҪәв________ЈЁМоЎ°УТТЖЎұ Ў°ЧуТЖЎұ»тЎ°І»ТЖ¶ҜЎұЈ©ЎЈ